Abstract
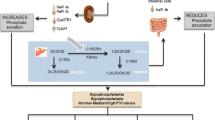
Investigation of X-linked hypophosphatemia (XLH) has led to the identification of a novel phosphate-regulating homeostatic system. Initially considered vitamin D-refractory rickets, renal phosphate wasting was identified as the cardinal biochemical feature of XLH and several related disorders. Current therapy employs calcitriol and phosphate, which usually improves, but does not completely heal deformities and short stature. Later complications of XLH include development of osteophytes, entheses, and osteoarthritis. The mutated gene in XLH, PHEX, is expressed in osteocytes, but its role in the pathogenesis of phosphate wasting is poorly understood. Many hypophosphatemic disorders are mediated by FGF23, a unique fibroblast growth factor with endocrine properties. Renal action of FGF23 leads to reduced expression of type II sodium-phosphate co-transporters, as well as reduced expression of CYP27B1, which encodes vitamin D 1α-hydroxylase. FGF23-mediated hypophosphatemia is characterized by inappropriately normal circulating 1,25-dihydroxyvitamin D together with renal phosphate wasting. The FGF23 system serves as a novel mechanism by which the mineralizing skeleton can communicate phosphate supply to the kidney and thereby mediate excretion or conservation of this important skeletal component. Other forms of FGF23-mediated hypophosphatemia represent various aberrations in this axis. Secretion of excess FGF23 (as in tumor-induced osteomalacia), and mutations preventing proteolytic cleavage of FGF23 result in similar clinical features. Other hypophosphatemic disorders are discussed.
Similar content being viewed by others
References
Winters RW, Graham JB, Williams TF, McFalls VW, Burnett CH (1958) A genetic study of familial hypophosphatemia and vitamin D-resistant rickets with a review of the literature. Medicine 37:97
Francis F (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet 11:130–136
Ruchon AF, Marcinkiewicz M, Siegfried G, Tenenhouse HS, DesGroseillers L, Crine P, Boileau G (1998) Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J Histochem Cytochem 46:459–468
Sabbagh Y, Boileau G, DesGroseillers L, Tenenhouse HS (2001) Disease-causing missense mutations in the PHEX gene interfere with membrane targeting of the recombinant protein. Hum Mol Genet 10:1539–1546
Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, Schiavi SC (2001) FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun 284:977–981
Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98:6500–6505
Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA (2005) Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin D metabolism in mice. Endocrinology 146:5358–5364
Antoniucci DM, Yamashita T, Portale AA (2006) Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 91:3144–3149
Perwad F, Zhang MY, Tenenhouse HS, Portale AA (2007) Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol 293:F1577–F1583
Strom TM, Francis F, Lorenz B, Böddrich A, Econs MJ, Lehrach H, Meitinger T (1997) Pex gene deletions in Gy and Hyp mice provide mouse models for X-linked hypophosphatemia. Hum Mol Genet 6:165–171
White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ (2001) Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60:2079–2086
White KE (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26:345–348
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
Ranch D, Zhang MY, Portale AA, Perwad F (2011) Fibroblast growth factor 23 regulates renal 1, 25-dihydroxyvitamin D and phosphate metabolism via the MAP kinase signaling pathway in Hyp mice. J Bone Miner Res 26:1883–1890
Xiao L, Naganawa T, Lorenzo J, Carpenter TO, Coffin JD, Hurley MM (2010) Nuclear isoforms of fibroblast growth factor 2 are novel inducers of hypophosphatemia via modulation of FGF23 and KLOTHO. J Biol Chem 285:2843–2846
Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD (2011) Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J 25:2551–2562
Yoshida T, Fujimori T, Nabeshima Y-I (2002) Mediation of unusually high concentrations of 1,25-dihydroxyvitamin D in homozygous klotho mutant mice by increased expression of renal 1α-hydroxylase gene. Endocrinology 143:683–689
Brownstein CA, Adler F, Nelson-Williams C, Iijima J, Li P, Imura A, Nabeshima Y, Reyes-Mugica M, Carpenter TO, Lifton RP (2008) A translocation causing increased alpha-klotho level results in hypophosphatemic rickets and hyperparathyroidism. Proc Natl Acad Sci USA 105:3455–3460
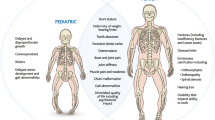
Reid IR, Hardy DC, Murphy WA, Teitelbaum SL, Bergfeld MA, Whyte MP (1989) X-linked hypophosphatemia: a clinical, biochemical, and histopathologic assessment of morbidity in adults. Medicine 68:336–352
Marie PJ, Glorieux FH (1982) Bone histomorphometry in asymptomatic adults with hereditary hypophosphatemic vitamin D-resistant osteomalacia. Metab Bone Dis Relat Res 4:249–253
Marie PJ, Glorieux FH (1981) Histomorphometric study of bone remodeling in hypophosphatemic vitamin D-resistant rickets. Metab Bone Dis Relat Res 3:31–38
Abe K, Ooshima T, Lily TS, Yasufuku Y, Sobue S (1988) Structural deformities of deciduous teeth in patients with hypophosphatemic vitamin D-resistant rickets. Oral Surg Oral Med Oral Pathol 65:191–198
Hillmann G, Geurtsen W (1996) Pathohistology of undecalcified primary teeth in vitamin D-resistant rickets: review and report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 82:218–224
Seeto E, Seow WK (1991) Scanning electron microscopic analysis of dentin in vitamin D-resistant rickets—assessment of mineralization and correlation with clinical findings. Pediatr Dent 13:43–48
Goodman JR, Gelbier MJ, Bennett JH, Winter GB (1998) Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int J Paediatr Dent 8:19–28
Liang G, Katz LD, Insogna KL, Carpenter TO, Macica CM (2009) Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int 85:235–246
Liang G, Vanhouten J, Macica CM (2011) An atypical degenerative osteoarthropathy in Hyp mice is characterized by a loss in the mineralized zone of articular cartilage. Calcif Tissue Int 89:151–162
Megerian CA, Semaan MT, Aftab S, Kisley LB, Zheng QY, Pawlowski KS, Wright CG, Alagramam KN (2008) A mouse model with postnatal endolymphatic hydrops and hearing loss. Hear Res 237:90–105
Lorenz-Depiereux B, Guido VE, Johnson KR, Zheng QY, Gagnon LH, Bauschatz JD, Davisson MT, Washburn LL, Donahue LR, Strom TM, Eicher EM (2004) New intragenic deletions in the Phex gene clarify X-linked hypophosphatemia-related abnormalities in mice. Mamm Genome 15:151–161
Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL (2011) A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res 26:1381–1388
Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, Shimada T (2009) Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res 24:1879–1888
Goetz R, Nakada Y, Hu MC, Kurosu H, Wang L, Nakatani T, Shi M, Eliseenkova AV, Razzaque MS, Moe OW, Kuro-o M, Mohammadi M (2010) Isolated C-terminal tail of FGF23 alleviates hypophosphatemia by inhibiting FGF23–FGFR–Klotho complex formation. Proc Natl Acad Sci USA 107:407–412
Yuan B, Bowman S, Meudt J, Blank R, Feng J, Drezner M (2010) Hexa-d-arginine reversal of osteoblast 7B2 dysregulation in Hyp-mice normalizes the HYP biochemical phenotype. J Bone Miner Res 25:S4
Yuan B, Meudt J, Blank R, Lindberg I, Drezner M (2011) Mechanism of hexa-d-arginine curative effects on the HYP phenotype. J Bone Miner Res 26:S90
Liu ES, Carpenter TO, Gundberg CM, Simpson C, Insogna KL (2011) Calcitonin administration in X-linked hypophosphatemia. N Engl J Med 364:1678–1680
Seikaly MG, Brown R, Baum M (1997) The effect of recombinant human growth hormone in children with X-linked hypophosphatemia. Pediatrics 100:879–884
Haffner D, Wuhl E, Blum WF, Schaefer F, Mehls O (1995) Disproportionate growth following long-term growth hormone treatment in short children with X-linked hypophosphataemia. Eur J Pediatr 154:610–613
Patel L, Clayton PE, Brain C, Pelekouda E, Addison GM, Price DA, Mughal MZ (1996) Acute biochemical effects of growth hormone treatment compared with conventional treatment in familial hypophosphataemic rickets. Clin Endocrinol 44:687–696
Carpenter TO, Keller M, Schwartz D, Mitnick M, Smith C, Ellison A, Carey D, Comite F, Horst R, Travers R, Glorieux FH, Gundberg CM, Poole AR, Insogna KL (1996) 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets—a clinical research center study. J Clin Endocrinol Metab 81:2381–2388
Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD (2008) Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol 3:658–664
Alon U, Chan JC (1985) Effects of hydrochlorothiazide and amiloride in renal hypophosphatemic rickets. Pediatrics 75:754–763
Weidner N, Santa Cruz D (1987) Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 59:144–154
Chong WH, Molinolo AA, Chen CC, Collins MT (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18:R53–R77
Bergwitz C, Collins MT, Kamath RS, Rosenberg AE (2011) Case records of the Massachusetts General Hospital. Case 33-2011. A 56-year-old man with hypophosphatemia. N Engl J Med 365:1625–1635
Harrison HE, Harrison HC (1979) Rickets and osteomalacia. In: Disorders of calcium and phosphate metabolism in childhood and adolescence. W.B. Saunders Company, Philadelphia, pp 141–256
Econs M, McEnery P (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate wasting disorder. J Clin Endocrinol Metab 82:674–681
Imel EA, Hui SL, Econs MJ (2007) FGF23 concentrations vary with disease status in autosomal dominant hypophosphatemic rickets. J Bone Miner Res 22:520–526
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ (2011) Iron modifies plasma fgf23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab 96:3541–3549
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE (2011) Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA 108:E1146-E1155 (Epub ahead of print)
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM (2006) DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38:1248–1250
Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86:267–272
Levy-Litan V, Hershkovitz E, Avizov L, Leventhal N, Bercovich D, Chalifa-Caspi V, Manor E, Buriakovsky S, Hadad Y, Goding J, Parvari R (2010) Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am J Hum Genet 86:273–278
Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Höhne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, Epplen JT, Knisely A, Superti-Furga A, McGill J, Filippone M, Sinaiko AR, Vallance H, Hinrichs B, Smith W, Ferre M, Terkeltaub R, Nürnberg P (2003) Mutations in ENPP1 are associated with ‘idiopathic’ infantile arterial calcification. Nat Genet 34:379–381
Dumitrescu CE, Collins MT (2008) McCune–Albright syndrome. Orphanet J Rare Dis 3:12
Sethi SK, Hari P, Bagga A (2010) Elevated FGF-23 and parathormone in linear nevus sebaceous syndrome with resistant rickets. Pediatr Nephrol 25:1577–1578
Konishi K, Nakamura M, Yamakawa H, Suzuki H, Saruta T, Hanaoka H, Davatchi F (1991) Hypophosphatemic osteomalacia in von Recklinghausen neurofibromatosis. Am J Med Sci 301:322–328
White KE, Cabral JM, Davis SI, Fishburn T, Evans WE, Ichikawa S, Fields J, Yu X, Shaw NJ, McLellan NJ, McKeown C, Fitzpatrick D, Yu K, Ornitz DM, Econs MJ (2005) Mutations that cause osteoglophonic dysplasia define novel roles for FGFR1 in bone elongation. Am J Hum Genet 76:361–367
Tieder M, Modai D, Samuel R, Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA (1985) Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med 312:611–617
Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Juppner H (2006) SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78:179–192
Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2006) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201
Xia W-B (2011) Clinical features and genetic analysis of patients with hypophosphatemic rickets. Japanese Bone and Mineral Society, Osaka
Jaureguiberry G, Carpenter TO, Forman S, Jüppner H, Bergwitz C (2008) A novel missense mutation in SLC34A3 that causes hereditary hypophosphatemic rickets with hypercalciuria in humans identifies threonine 137 as an important determinant of sodium-phosphate cotransport in NaPi-IIc. Am J Physiol Renal Physiol 295:F371–F379
Magen D, Berger L, Coady MJ, Ilivitzki A, Militianu D, Tieder M, Selig S, Lapointe JY, Zelikovic I, Skorecki K (2010) A loss-of-function mutation in NaPi-IIa and renal Fanconi’s syndrome. N Engl J Med 362:1102–1109
Scheinman SJ (1998) X-linked hypercalciuric nephrolithiasis: clinical syndromes and chloride channel mutations. Kidney Int 53:3–17
Karim Z, Gérard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prié D (2008) NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359:1128–1135
Conflict of interest
Dr. Carpenter has consulted for and receives grant support from Kyowa Hakko Kirin Co., and serves on a Data Safety and Monitoring Board for Enobia, Inc.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Carpenter, T.O. The expanding family of hypophosphatemic syndromes. J Bone Miner Metab 30, 1–9 (2012). https://doi.org/10.1007/s00774-011-0340-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-011-0340-2