Abstract
In this article a brief version of the newly developed S3 guidelines on screening, diagnosis, therapy and follow-up of abdominal aortic aneurysms (AAA), which was developed under the auspices of the German Society of Vascular Surgery and VascularMedicine (DGG) is presented. In addition to the DGG, participating professional societies were the German Radiological Society (DRG), German Society for Angiology/Society for Vascular Medicine (DGA), German Interdisciplinary Association for Intensive Care and Emergency Medicine (DIVI), German Society for Ultrasound in Medicine (DEGUM), German Vascular League e.V., German Society for Interventional Radiology (DEGIR), German Society for Anesthesiology and Intensive Care Medicine (DGAI), German Society for Thoracic and Cardiovascular Surgery (DGTHG) and the German Society of Surgery (DGCH). The guidelines are based on a systematic literature search in Medline (PubMed) for the period from 1 January 2000 to 1 January 2017. Important publications from 2017 were also considered. The selection of evidence was made by a multilevel screening process. In addition to the evidence, other criteria were included in the assessment of the recommendation level, such as the consistency of the study results, the clinical relevance of the endpoints and effect sizes, the benefit–risk balance, the applicability of the study results to the patient target group and the care system, the feasibility of the recommendations in everyday life, patient preferences and ethical and legal considerations. All recommendations/findings were approved with strong consensus (approval of >95% of the participating professional societies/associations). Thus, the present text version of the S3 guidelines on AAA represents the view of all participating societies.
Zusammenfassung
Die unter Federführung der Deutschen Gesellschaft für Gefäßchirurgie und Gefäßmedizin (DGG) neu erarbeitete S3-Leitlinie zu Screening, Diagnostik, Therapie und Nachsorge des Bauchaortenaneurysmas (AAA) wird in Kurzfassung vorgestellt. Beteiligte Fachgesellschaften waren neben der DGG die Deutsche Röntgen-Gesellschaft (DRG), Deutsche Gesellschaft für Angiologie/Gesellschaft für Gefäßmedizin (DGA), Deutsche Interdisziplinäre Vereinigung für Intensiv- und Notfallmedizin (DIVI), Deutsche Gesellschaft für Ultraschall in der Medizin (DEGUM), Deutsche Gefäßliga e. V., Deutsche Gesellschaft für Interventionelle Radiologie (DEGIR), Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), Deutsche Gesellschaft für Thorax‑, Herz- und Gefäßchirurgie (DGTHG) und die Deutsche Gesellschaft für Chirurgie (DGCH). Die Leitlinie beruht auf einer systematischen Literaturrecherche in Medline (PubMed) für den Zeitraum vom 01.01.2000 bis 01.01.2017. Wichtige Publikationen des Jahres 2017 wurden ebenfalls berücksichtigt. Die Auswahl der Evidenz erfolgte durch einen mehrstufigen Screeningprozess. In die Bewertung des Empfehlungsgrades gingen neben der Evidenz weitere Kriterien ein, wie die Konsistenz der Studienergebnisse, die klinische Relevanz der Endpunkte und Effektstärken, das Nutzen-Risiko-Verhältnis, die Anwendbarkeit der Studienergebnisse auf die Patientenzielgruppe und das Versorgungssystem, die Umsetzbarkeit der Empfehlungen im Alltag, Patientenpräferenzen und ethische und rechtliche Gesichtspunkte. Alle Empfehlungen/Feststellungen wurden mit „starkem Konsens“ verabschiedet (Zustimmung von >95 % der teilnehmenden Fachgesellschaften/-verbände). Somit repräsentiert die vorliegende Textfassung der S3-Leitlinie Bauchaortenaneurysma die Ansicht aller beteiligten Fachgesellschaften.
Similar content being viewed by others
On 3 July 2018, under the leadership of the German Society for Vascular Surgery and Vascular Medicine (DGG), the new S3 guidelines for screening, diagnosis, treatment, and follow-up for abdominal aortic aneurysms (AAA) were adopted by the DGG Executive Board, after unanimous approval by all participating professional associations. A total of 10 medical societies participated in the preparation of the guidelines, now available at awmf-online.de. These guidelines are unique in being the only interdisciplinary guidelines for AAA, covering not only screening, diagnostics, indications for surgery, vascular surgery/interventional therapy, and follow-up care but also describing perioperative and intensive care management. Other guidelines, as for example, the newly created guidelines of the Society for Vascular Surgery (SVS; [1]), are not as comprehensive. Given the considerable scope of the DGG guidelines, a short version is presented here, including all assertions and recommendations with a brief justification.
Methods
The guidelines are based on a systematic literature search in Medline (PubMed) from 1 January 2000 to 1 January 2017 and important publications from 2017 have also been considered. A multistage screening process was employed to select evidence. First, all existing guidance relevant to clinical issues, considered important (key questions) by the Guideline Steering Group, was analyzed. In a second step, all available Cochrane reviews on AAA were subjected to an evaluation algorithm. Systematic reviews and meta-analyses were also subjected to an evaluation algorithm and then analyzed with respect to answering unanswered or insufficiently answered key questions. To answer still insufficiently answered or unanswered key questions, therapeutic and diagnostic studies from the primary literature were consulted.
Evidence evaluation was carried out with the aid of structured checklists. In addition to the evidence, other criteria were included in evaluating the level of recommendation. These incorporated consistency of study results, the clinical relevance of endpoints and effect sizes, the benefit-risk balance, the applicability of study results to patient target group and healthcare system, the feasibility of everyday recommendations, patient preferences, and ethical and legal considerations. The level of recommendation followed the Association of the Scientific Medical Societies in Germany (AWMF) guidance manual and rules for guideline development (Table 1).
A total of three recommendation levels are differentiated and expressed by the words “strongly recommended”, “recommended (should)” and “recommendation open (can)”. Recommendations against intervention are accordingly expressed as “strongly not recommended”, “should not be recommended”, or “can be dispensed with”. As a rule, the quality of the level of evidence determines the degree of recommendation. This means that a recommendation based on an average level of evidence is usually associated with a middle recommendation grade. Recommendations depend on the available evidence and recommendations with missing or incomplete evidence are listed as consensus recommendations as a result of interdisciplinary discussion (GCP = good clinical practice). The recommendation levels are based on specifications of the Oxford Center for Evidence-based Medicine (Table 2).
All assertions/recommendations were adopted with strong consensus (approved by >95% of the participating professional societies/associations). Thus, the present text version of the S3 Guidelines on Abdominal Aortic Aneurysms represents the view of all participating societies. In presenting the consensus for the short version of these guidelines, individual recommendations were waived to avoid redundancy.
Assertions and recommendations
1. Definition AAA
Assertion.
An AAA is defined as an abdominal aortic diameter ≥ 30 mm, either in the anteroposterior or transverse plane.
2.1 AAA prevalence/incidence
Assertion.
For men initially invited to screening in England and Sweden, the prevalence of AAA was 1.3–1.7%. Level of evidence 2b.
2.2 Risk factors for developing an AAA
Assertion.
In addition to age, the two major risk factors for developing an AAA are male gender (odds ratio 5.69) and smoking (odds ratio 2.41). Level of evidence 2a.
2.3 Rupture risk
Assertion.
The rupture risk for small aneurysms (3.0–5.5 cm) ranges between 0 and 1.61/100 person-years. Level of evidence 1b.
Assertion.
The pooled annual rupture risk for patients not fit for surgery is 3.5% for AAA sized from 5.5 cm to 6.0 cm, 4.1% for AAA from 6.1 cm to 7.0 cm, and 6.3% for AAA > 7.0 cm. Level of evidence 2b.
2.4 Optimizing risk factors and pharmacotherapy
Assertion.
Along with peripheral arterial occlusive disease (PAOD), AAA is the cardiovascular disease most strongly associated with smoking. Level of evidence 2a.
Recommendation.
Physicians should strongly recommend that patients give up smoking, particularly after treatment for an AAA. Level of evidence 2a/recommendation grade A.
Reason.
Smoking is the most important modifiable risk factor for AAA development. In the Southern Community Cohort study in the USA with 18,782 participants aged ≥65 years, the AAA age-adjusted incidence per 100,000 men was 198.5 for never smokers, 369.4 for former smokers, and 563.3 for current smokers. Comparative figures for women were 50.8 (never smokers), 225.2 (former smokers), and 843.4 (current smokers) [2]. It is therefore essential to advise AAA patients to give up smoking. In the Atherosclerosis Risk in Communities (ARIC) study [3] 15,792 participants were recruited between 1987 and 1989 and followed until 2013. Reviewing their smoking status between visit 1 (1987–1989) and visit 4 (1996–1998) revealed that smokers who quit smoking between visits 1 and 4 had a 29% lower lifetime risk of developing an AAA than those who continued to smoke. Nevertheless, the risk was still higher than that of participants who had already given up smoking before the first visit.
Recommendation.
Blood pressure control and hypertension treatment is strongly recommended in all patients with AAA to prevent cardiovascular complications. Level of evidence 1a/recommendation grade A.
Reason.
Blood pressure control is strongly recommended to prevent cardiovascular disease in all patients with AAA; however, it is highly questionable whether treatment for hypertension is beneficial beyond preventing cardiovascular morbidity by significantly influencing the AAA expansion rate. A systematic review of the literature revealed that hypertension is associated with the presence of AAA but there is no evidence that the AAA expansion rate is higher in hypertensive patients [4]. Accordingly, there is no evidence supporting a beneficial effect of beta blockers on AAA expansion rate [5].
Recommendation.
If there are no contraindications, it is strongly recommended that patients with AAA and cardiovascular comorbidities receive statins for cardiovascular protection. Level of evidence 2a/recommendation grade A.
Reason.
A systematic review with a meta-analysis based on 12 cohort studies (11 retrospective, 1 prospective) was concerned with the influence of statin treatment on expansion rate and mortality connected with AAA [6]. Randomized studies were not available. A sensitivity analysis failed to demonstrate any influence of statin treatment on AAA expansion rate. Similarly, there was no significant difference in the 30-day mortality after AAA treatment between those patients receiving and not receiving statins; however, patients receiving statin therapy experienced significantly lower mortality 1, 2, and 5 years after aneurysm treatment, a trend that increased with treatment duration.
Recommendation.
Preprocedural initiation of statin therapy should be considered in patients undergoing vascular surgery, ideally at least 2 weeks before the intervention. Level of evidence 2a/recommendation grade B.
Reason.
Twine and Williams [6] concluded in their meta-analysis that there is no solid evidence that AAA patients perioperatively benefit from statin treatment. Notwithstanding, based on the randomized DECREASE III study, perioperative statin treatment compared to placebo resulted in a significant reduction in myocardial ischemia connected with vascular interventions (47.5% of these interventions were on the abdominal aorta) [7]. An evaluation of a Medicare database with 19,323 patients (14,602 endovascular and 4721 open AAA interventions) showed that approximately half received preprocedural statins. Patients receiving statins had significantly lower mortality after 90 days and during the first year after the intervention [8].
Recommendation.
Those AAA patients with cardiovascular comorbidities should be advised to use anticoagulants. Level of evidence 2a/recommendation grade B.
Reason.
Randomized studies concerning the influence of anticoagulants on AAA patient mortality and the incidence of cardiovascular events do not exist [9]; however, as with other atherosclerotic diseases, anti-coagulant treatment is generally recommended to prevent cardiovascular events in patients with AAA [10]. While in the English cohort study by Bahia et al. [11] patients on anti-coagulant treatment showed significantly better survival but this did not hold true in the post hoc analysis of data stemming from the DREAM study [12].
Recommendation.
It is strongly recommended not to treat small AAAs with doxycycline. Level of evidence 2b/recommendation grade A.
Reason.
In a randomized, double-blind, placebo-controlled study Meijer et al. [13] reviewed whether 18-month doxycycline treatment can reduce AAA progression. Doxycycline did not reduce aneurysm growth, and growth was even slightly (but significantly) higher in the treated group than in the control group.
Recommendation.
Coronary revascularization should be performed prior to AAA intervention in patients with acute myocardial infarction with or without ST elevation, unstable or stable angina with left main stem or 3‑vessel disease. Evidence level 2b/recommendation grade B.
Reason.
The indications for preoperative coronary revascularization should follow established guidelines [14] and be limited to the abovementioned target groups.
3.1 AAA screening (early detection)
Recommendation.
Screening for AAA with ultrasound
-
is strongly recommended for all men >65 years. Level of evidence 1a/recommendation grade A.
-
is strongly recommended for women >65 years with a current or past history of smoking. Level of evidence 2a,/recommendation grade A.
-
should not be recommended for female non-smokers without a family AAA history. Level of evidence 2a/recommendation grade B.
-
should be considered for first-degree siblings of patients with AAA. Level of evidence 2c/recommendation grade B.
Reason.
-
AAA screening for men. An assessment of patient-relevant endpoints concerning benefits of ultrasound screening compared to no screening was carried out on behalf of the Joint Federal Committee (final report 2015) [15] by the Institute for Quality and Efficiency in Health Care (IQWIG) and four randomized controlled trials (RCTs) were included in the benefit assessment. For men, after 13–15 years of follow-up ultrasound screening was associated with a significant decrease in all-cause mortality, AAA-related mortality, rupture frequency, and emergency surgery. Screening was also associated with a significant increase in AAA-specific elective interventions. Quality of life results were not usable. The IQWIG final report thus confirmed earlier findings from a Cochrane review, which also concluded on the basis of these four RCTs that there is evidence that AAA ultrasound screening in men aged 65–79 years leads to a significant reduction of AAA-related mortality [16].
-
AAA screening for women. Cosford and Leng [16] failed to prove a benefit of AAA screening for women in their Cochrane review. The same applied to a meta-analysis carried out by IQWIG [15]; however, the lack of evidence of benefit from AAA screening in women does not mean that benefit may not be obtained with longer follow-up in women with a high AAA risk, especially in smokers >65 years. In a study from the USA [2], the risk for active smokers for developing AAA was ninefold higher than for non-smokers (hazard ratio 9.17). A meta-analysis examining AAA prevalence in screened women aged ≥60 years, was presented by Ulug et al. [17]. In this systematic review 8 studies were published by January 2016, with a total of 1,537,633 screened women. The AAA prevalence rates were very heterogeneous, ranging from 0.37% to 1.53% and the pooled prevalence was 0.74%. The pooled prevalence increased with age (more than 1% in women ≥70 years). The same was true for smokers (>1% in women who ever smoked and >2% in active smokers). Consequently, anyone planning a screening program for all men aged 65 years should also invite women who smoke to AAA screening.
-
AAA screening of first-degree relatives. First-degree relatives of AAA patients represent a target group for AAA screening. In the Aneurysm Detection and Management study (ADAM) [18], family history after smoking was the second highest risk factor for development of an AAA (odds ratio 1.94). This observation was confirmed in a population-based Swedish case-control study [19], in which the risk of developing an AAA was doubled for first-degree relatives of persons diagnosed with AAA, compared to persons without a corresponding family history.
Assertion.
A prerequisite to screening success is low periprocedural morbidity and mortality of elective AAA repair in screened patients, since the rupture risk for small aneurysms (under 5.5 cm) in men is 0–1.61/100 person-years. Level of evidence 1b.
3.2 Surveillance of small aneurysms
Recommendation.
Monitoring intervals for small asymptomatic AAA in men:
-
every 2 years for AAA with a diameter of 3.0–3.9 cm,
-
once a year for AAA with a diameter of 4.0–4.9 cm,
-
every 6 months for AAA with a diameter of 5.0–5.4 cm. Level of evidence 2a/recommendation grade A.
Recommendation.
Monitoring intervals for small asymptomatic AAA in women:
-
every 2–3 years for AAA with a diameter of 3.0–3.9 cm,
-
every 6 months for AAA with a diameter of 4.0–4.5 cm*,
-
every 3 months for AAA with a diameter >4.5–4.9 cm*.
*If the diameter remains constant, the interval can be extended. Level of evidence 3b/recommendation grade B.
Reason.
A systematic review and a meta-analysis of the literature [20, 21] have shown that monitoring intervals of small aneurysms should be tailored to the growth rate. Any increase in the aneurysm diameter of 0.5 cm increases aneurysm growth by 0.5 mm/year, with a doubling of the rupture risk. The authors calculated a pooled mean growth rate of 1.8 mm/year for aneurysms 3.0–4.4 cm in diameter, compared to 4.96 mm/year for AAA 4.5–4.9 cm in diameter. The growth rate in smokers is 0.35 mm/year higher than in ex-smokers or non-smokers. Conversely, the growth rate is slower by 0.51 mm/year in diabetics than in non-diabetics. The recommended monitoring intervals refer to AAA rescreening for men. Although similar AAA growth rates were observed in women, aneurysms in women are marked with a fourfold higher rupture risk. This leads to recommending monitoring intervals of 2–3 years for women with an AAA of 3.0–3.9 cm but to narrowing the monitoring intervals after this time.
3.3 Ultrasound techniques
Recommendation.
Perpendicular measurement at right angles to the longitudinal vessel axis is the leading-edge method and is strongly recommended for ultrasound screening. Level of evidence 3b/recommendation grade A.
Reason.
The B-mode ultrasound (real-time gray scale sonography) is the method of choice for screening. Ultrasound measurement of both aortic diameter and the measurement plane must be standardized to facilitate comparative examinations and valid follow-up examinations [22]. This leading-edge method has provided the most accurate results. Hereby, the distance between the outer wall reflection to the opposite inner wall reflection is measured, i. e. from the outer beginning of the echogenic wall reflection to the luminal reflection point on the opposite side.
4. Indications
4.1 Indications for treatment of asymptomatic AAA
Recommendation.
Treatment of asymptomatic AAA:
-
regular monitoring is strongly recommended as the first-line management strategy of choice for asymptomatic AAA 4.0–5.4 cm in size. Level of evidence 1a/recommendation grade A.
-
patients with an infrarenal or juxtarenal AAA ≥ 5.5 cm are strongly recommended to undergo elective interventions. Level of evidence 1a/recommendation grade A.
-
patients with an infrarenal or juxtarenal AAA of 5.0–5.4 cm can be considered for elective intervention. Level of evidence 3b/recommendation grade 0.
-
invasive interventions should be considered for women when the maximum AAA diameter has reached 5.0 cm. Level of evidence 3b/recommendation grade B.
-
regardless of AAA diameter, if the growth rate is >10 mm/year, an open repair (OR) or endovascular aortic aneurysm repair (EVAR) intervention is indicated and strongly recommended. Level of evidence 1a/recommendation grade A.
These recommendations apply to the fusiform aneurysm. No data are available for saccular AAAs; therefore, well-founded recommendations for saccular AAA cannot be made.
Reason.
A Cochrane review [23] on mortality, quality of life, and cost-effectiveness of immediate care versus routine ultrasound-guided monitoring in patients with asymptomatic AAA from 4.0 cm to 5.5 cm was based on a meta-analysis of 4 randomized trials with 3314 participants. The authors concluded from the pooled meta-analysis that current evidence supports postponing the time of intervention until the AAA reaches 5.5 cm in diameter. The results of the four studies show no advantage of immediately subjecting smaller AAAs to treatment, regardless of whether OR or EVAR is chosen. In the relevant randomized controlled trials, as a rule men were included due to the significantly higher prevalence of AAA in men. Thus, there is a lack of reliable data on the diameter limit mandating interventional AAA care in women. It must also be taken into account that women generally have a smaller aortic diameter than men but a fourfold increased risk of rupture [24]. Based on these data, recommendations are made to already set the limit indicator for women at 5.0 cm [25].
4.2 Indications for treatment of symptomatic AAA
Recommendation.
It is strongly recommended that patients with symptomatic AAA be subjected to an invasive intervention at the next possible elective date. Level of evidence 2b/recommendation grade A.
Recommendation.
Patients with symptomatic AAA should be treated endovascularly, if technically possible. Level of evidence 2b/recommendation grade B.
Reason.
Historically, symptomatic patients receiving urgent treatment have had a significantly higher clinical mortality than asymptomatic patients receiving elective care; however, this information requires revision. In a large recent study (Vascular Study Group of Northern New England) with 156 symptomatic AAAs, hospital mortality after symptomatic AAA treatment was 1.7%, which was hardly different from the 1.3% reported for elective asymptomatic care [26]. Using data from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) in 2011-2013, this result was further examined with respect to benefits of EVAR vs. OR [27]. Here, symptomatic patients were reported to have a twofold increased 30-day perioperative mortality risk, compared to asymptomatic patients, with the mortality risk being significantly higher with OR than with EVAR.
4.3 Indications for treatment of ruptured AAA
Recommendation.
A ruptured AAA (rAAA) is an emergency, which must immediately be treated invasively. Level of evidence 2b/recommendation grade A.
Recommendation.
In patients with successfully treated cardiac arrest (return of spontaneous circulation, ROSC), treatment may be justified. Level of evidence 4/recommendation grade 0.
Reason.
The rAAA is an emergency and immediate treatment is strongly recommended. An international comparison of US registry data (n = 23,838) with that of England (n = 11,799) [28] provides a unique insight into the care of unselected patients with rAAA. The comparison included all inpatients admitted with rAAA, regardless of whether interventions were carried out or not. Significantly fewer patients with rAAA died in the USA than in England. In the USA, 80.4% of patients were treated, compared with just over half in England (58.4%). The mortality of all patients admitted was 53.1% in the USA and 65.9% in England.
5. Preprocedural comorbidity and risk evaluation
5.1 Comorbidity
Assertion.
In patients with renal insufficiency requiring dialysis and in patients with chronic obstructive pulmonary disease (COPD) requiring oxygen, the benefit of elective intervention for AAA is not assured. Level of evidence 2a.
Reason.
Patient comorbidities significantly influence long-term survival after surgical treatment of an AAA. A systematic review (51 articles) and meta-analysis (45 studies) of long-term survival after AAA treatment over the last 25 years have shown renal insufficiency with a hazard ratio (HR) of 3.15 and COPD with oxygen therapy (HR 3.05) as the risk factors most limiting life expectancy [29].
5.2. Risk evaluation
Good clinical practice in clarifying cardiac risk
-
cardiac risk assessment using the revised cardiac risk index (RCRI),
-
determination of brain natriuretic peptide (BNP) or N-terminal pro-brain natriuretic peptide (NT-proBNP) in cardiac high risk patients (exercise tolerance <4 metabolic equivalents, MET, revised cardiac risk index, RCRI >1),
-
12-lead electrocardiograph (ECG) for anamnestic conspicuous or cardiac symptomatic patients,
-
non-invasive cardiac stress tests in patients with ≥3 clinical risk factors and limited (<4 MET) exercise capacity,
-
preoperative coronary angiography in patients with proven myocardial ischemia,
-
preoperative echocardiography in newly diagnosed dyspnea of unknown origin or in unknown heart failure and worsening of symptoms within the last 12 months.
Pulmonary risk
Recommendation.
A preprocedural lung function test should be performed in patients with suspected pulmonary disease. Level of evidence 3b/recommendation grade B.
Reason.
The guideline recommendation follows the joint recommendations of the German Society for Anesthesiology and Intensive Care Medicine, the German Society of Surgery and the German Society of Internal Medicine for the preoperative evaluation of adult patients before elective, non-cardiothoracic surgery [36].
Renal risk
Recommendation.
In patients with impaired renal function receiving endovascular AAA treatment, preinterventional and postinterventional hydration are strongly recommended to avoid contrast-related nephropathy. Level of evidence 1b/recommendation grade A.
Reason.
Serum creatinine and glomerular filtration rate (GFR) should be determined preoperatively in all AAA patients. All patients with a GFR < 45 ml/min/1.73 m2, i. e. from chronic kidney disease (CKD) stage 3b or with a GFR < 60 ml/min/1.73 m2, i. e. from CKD stage 3a and simultaneous occurrence of proteinuria, hematuria, hypertension, morphologic changes, or kidney-specific comorbidities should be referred to a nephrologist [30]. The most important measure to avoid contrast agent-induced nephropathy is adequate preoperative hydration, particularly when EVAR will be employed. For this purpose, it is recommended that the patient receives 0.9% saline solution i. v. at a rate of 1–1.5 ml/kg/h for 12 h prior to surgery and up to 24 h after surgery [31]. Furthermore, reference may be made to the guidelines of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS; [32]) as well as the guidelines of the European Society of Urogenital Radiology (ESUR, [33]).
Sonography of cervical vessels
Recommendation.
Patients with proven AAA should be preprocedurally examined by duplex sonography for stenotic internal carotid arteries. Level of evidence 4/recommendation grade B.
Reason.
Kurvers et al. [34] reported the prevalence of an internal carotid artery stenosis (ICAS) >70% in 8.8% of persons with AAA and recommended ICAS screening for AAA patients. In another study, the ICAS prevalence in patients with AAA was calculated at 10.8%, as a consequence of which preprocedural duplex sonography was recommended [35]. Reference can be made to the joint recommendation of the German Society for Anesthesiology and Intensive Care Medicine, the German Society of Surgery and the German Society of Internal Medicine for the preoperative evaluation of adult patients prior to elective, non-cardiothoracic surgery [36].
Risk indices
Recommendation.
For preprocedural risk assessment EVAR vs. OR, the BAR score may be used. Level of evidence 3b/recommendation grade 0.
Reason.
The mortality risk for EVAR or OR for an individual patient can be calculated in less than 30 s via the BAR score calculator (www.britishaneurysmrepairscore.com), which can be used to provide patient counselling on the risk of EVAR or OR elective intervention [37, 38].
6. Preprocedural diagnostic imaging
Recommendation.
For preprocedural diagnostic AAA imaging, computed tomography angiography (CTA) is strongly recommended as the method of choice. Level of evidence 3a/recommendation grade A.
Recommendation.
It is strongly recommended that non-contrast enhanced magnetic resonance angiography (MRA) be used as an alternative to CTA to avoid radiation exposure, especially in patients with impaired renal function. The MR compatibility of the implants must previously be checked. Level of evidence 3a/recommendation grade A.
Reason.
The use of CTA, including 3D vessel reconstruction, is the imaging tool of choice for precise preoperative planning [39]. The CTA should be performed from the head to below the groin as thin-layer computer tomography (CT slice thickness ≤ 1 mm) to depict accompanying aneurysms and access routes. The CTA delivers the highest predictive accuracy for suspected AAA rupture [40] and CT shows the extent of wall calcification better than any other imaging modality. Parietal thrombosis can also be very accurately demarcated. Not infrequently, CT reveals additional findings (e.g. basal pulmonary nodules, solid renal tumors) relevant for prognosis or requiring further clarification. A disadvantage is the contrast administration in patients with impaired renal function, iodine contrast allergy, or hyperthyroidism.
Extended diagnostics
Recommendation.
It is strongly recommended that patients with proven AAA be examined with duplex sonography for popliteal aneurysms. Level of evidence 4/recommendation grade A.
Reason.
Femoral artery aneurysms and popliteal artery aneurysms are common in patients with AAA. Diwan et al. [41] reported the prevalence of femoral and popliteal aneurysms in patients with AAA as 12%, in men as 14% with only 10/51 aneurysms being palpable.
Assertion.
Approximately 1 in every 20 patients scheduled for an AAA intervention has a malignancy. Level of evidence 2b.
Recommendation.
It is strongly recommended that patients with AAA be subjected to diagnostic imaging, including the thorax, prior to invasive treatment (preferably CT). Level of evidence 2b/recommendation grade A.
Diagnostics for rAAA
Recommendation.
In hemodynamically stable patients with signs of rAAA, it is strongly recommended to employ emergency CT. Level of evidence 3b/recommendation grade A.
Recommendation.
It is strongly recommended that hemodynamically unstable patients with confirmed AAA rupture be brought directly into the (hybrid) operating room. Level of evidence 3b/recommendation grade A.
Reason.
In its position paper the World Society of Emergency Surgery (WSES; [42]) recommended planned endovascular care to proceed in accordance with the protocol of Mehta et al. [115]. This means performing an emergency CT in stable patients (systolic blood pressure > 80 mm Hg). Hemodynamically unstable patients (systolic blood pressure < 80 mm Hg) should be brought to the operating room immediately. Under provision of an occlusion balloon for the supraceliac aorta, digital subtraction angiography (DSA) on the operating table should be used to evaluate whether the aneurysm neck is suitable for endovascular surgery or not.
7. Treatment choice—intact AAA (iAAA)
Recommendation.
For patients with acceptable periprocedural risk, EVAR or OR are equally recommendable, assuming anatomical feasibility for EVAR. Level of evidence 1a/recommendation grade A.
Recommendation.
Patient preference should strongly be considered when selecting the interventional procedure, taking into account differences between EVAR and OR in the periprocedural course, re-intervention frequency, follow-up and long-term aneurysm-related mortality. Level of evidence 1a/recommendation grade A.
Assertion.
Up to 1 year after intervention, EVAR is considered to provide benefits for the quality of life over OR. Level of evidence 3a.
Reason.
Important, randomized, prospective studies comparing EVAR and OR have been performed under the labels EVAR 1 (England), DREAM (Netherlands), OVER (USA), and ACE (France). Based on these four RCTs and two registry surveys, Stather et al. [43] compiled a systematic literature review and meta-analysis concerning the long-term outcome of EVAR vs. OR. The EVAR patients were found to have a significantly lower in-hospital mortality/30-day mortality compared to OR (1.3% vs. 4.7%); however, after 2 years, no differences between the 2 procedures (total mortality: EVAR 14.3% vs. OR 15.2%) or for aneurysm-related mortality (EVAR 3.9%, OR 4.5%) were apparent and after 4 years no significant differences were observed either (mortality after EVAR 34.7%, after OR 33.8%). Re-intervention rates were significantly higher for EVAR than for OR in the randomized trials (26.2% vs. 14%, respectively) and in the analysis of all patients included (EVAR 28.9% vs. OR 25.5%). An identical conclusion was reached in a Cochrane review based on the four RCTs and another survey [44].
Laparoscopic AAA treatment
Assertion.
The value of laparoscopic AAA treatment is unclear. Level of evidence 4.
Treatment in centers
Recommendation.
It is strongly recommended that AAA be treated in specialized centers. Level of evidence 2a/recommendation grade A.
Reason.
It is generally recommended to treat AAAs only in centers with sufficient experience. This requirement is broadly accepted and well-founded [45], since a clear relationship between hospital capacity, case size of individual surgeons, and outcome is apparent; however, no definitive volume limits can be established as evidenced by a systematic review of the literature [46]. Hospital size and structure may have a more significant impact on outcome than the absolute number of cases [47]. A review of 16 studies showed a relationship between hospital case load for AAA care and hospital mortality [48]. A meta-analysis of 32 publications, including the UK hospital episode statistics [49], also addressed the relationship between case load and quality of AAA care. According to this, the threshold for hospitals with low and high caseloads for AAA elective care is 43 interventions/year, with significantly better results in high-volume hospitals (odds ratio 0.66). For elective, conventional, open AAA interventions in the USA, Birkmeyer et al. [50] reported an adjusted operating mortality for surgeons with less than 8 interventions/year at 6.2%, for 8–17.5 interventions/year at 4.6%, and for more than 17.5 interventions/year at 3.9%.
Accompanying iliac aneurysms
Recommendation.
It is strongly recommended to be aware that iliac aneurysms >3.0 accompanying AAAs that require treatment, often constitute a requisite for extending the procedure. Level of evidence 4/recommendation grade A.
Reason.
Iliac aneurysms < 3.0 cm in diameter almost never rupture [51]. In a study by Santilli et al. [52] ruptures were observed only at a diameter >4 cm, Huang et al. [53] observed no ruptures in aneurysms < 3.8 cm.
8. Perioperative measures and techniques for intact AAA (iAAA)—OR
8.1 Perioperative management
Recommendation.
It is strongly recommended that patients receiving a vascular prosthesis for AAA repair receive systemic perioperative antibiotic prophylaxis against staphylococci and gram-negative bacteria. Level of evidence 2a/recommendation grade A.
Reason.
A Cochrane review clearly demonstrated the benefit of perioperative antibiotic prophylaxis in reducing wound and prosthetic infection rates for peripheral arterial reconstruction [54].
Recommendation.
Since perioperative hypothermia can impair the surgical result and negatively influence the postoperative course, it is strongly recommended to actively prevent hypothermia. Level of evidence 2a/recommendation grade A.
Reason.
Interdisciplinary S3 guidelines [55] that were developed to prevent perioperative hypothermia by, among others, the German Society for Anesthesiology and Intensive Care Medicine and the German Society for Surgery should be referred to.
Assertion.
Currently available data concerning intraoperative fluid management speaks against administering fluids with supraphysiological chloride concentrations. Level of evidence 3a.
Assertion.
A target-oriented volume therapy reduces the complication rate in high-risk patients. Level of evidence 2b.
Recommendation.
When greater blood loss is anticipated during AAA interventions, it is strongly recommended that blood management be implemented, including automated autotransfusion. Level of evidence 2a/recommendation grade A.
Reason.
A joint recommendation was presented by the German Society for Anesthesiology and Intensive Care Medicine and the German Society for Surgery to implement patient blood management (PBM; [56]), which also applies to patients with AAA.
Assertion.
Fast-track protocols shorten in-hospital time. Level of evidence 3b.
Recommendation.
Based on an individual benefit-risk assessment, combining general with epidural anesthesia may be considered in open elective AAA repair. Level of evidence 3a/recommendation grade 0.
Reason.
Regional spinal anesthesia requires a special risk-benefit assessment due to the risk of spinal/epidural hematoma. If anticoagulation is interrupted, the individual patient-specific risk of thromboembolic and ischemic complications must be considered [57]. In a systematic review and meta-analysis of randomized, controlled studies, Pöpping et al. [58] investigated the effects on mortality and morbidity of a combined epidural-general anesthesia (EA-GA) vs. general anesthesia (GA) alone. A total of 125 studies involving 9044 patients were included, 657 of whom underwent vascular surgery and 10 studies (2201 patients) contained mortality data as a primary or secondary endpoint. The odds ratio for mortality risk in the EA-GA cohort was 0.60 (95% confidence interval, CI, 0.39–0.93) or 0.66 (95% CI, 0.42–1.02), depending on the inclusion or exclusion of 1 critically evaluated study. The 10 studies, including the 657 patients undergoing vascular surgery, favored epidural anesthesia (odds ratio 0.39, 95% CI 0.17–0.88). The mortality rate was 2.5% (9/353 patients) in the EA-GA cohort vs. 5.3% (16/304 patients) in the control group.
The results of all randomized controlled trials comparing postoperative epidural analgesia to postoperative systemic opioid-based analgesia in patients undergoing elective open abdominal aortic surgery were analyzed in a Cochrane review (15 studies, 1498 patients) [59]. Most of the studies evaluated thoracic epidural anesthesia but studies evaluating lumbar epidural anesthesia were also included. There was moderate evidence of reduced rates of myocardial infarction and postoperative respiratory failure, and a shortened length of intensive care stay with epidural anesthesia. There were, however, no differences in 30-day mortality.
Recommendation.
Postoperatively, the AAA patient treated by OR should be moved to an intermediate care (IMC) or intensive care unit (ICU). Level of evidence 4/recommendation grade B.
Reason.
The ICU postoperative care is generally indicated for patients with significant cardiac, pulmonary, or renal comorbidities, and for those in need of postoperative ventilation, or those developing significant arrhythmia or hemodynamic instability during surgery. There are no specific recommendations for postoperative monitoring of patients after AAA repair. Accordingly, general aspects of postanesthesia surveillance and intensive care apply. Existing recommendations, such as basic monitoring and extended hemodynamic monitoring, can be adapted for the AAA patient. Reference is made to the generally transferable S3 guideline recommendations on intensive care for cardiac surgery patients regarding hemodynamic and cardiovascular monitoring [60], or for volume replacement in intensive care patients in the S3 guidelines for intravascular volume therapy in adults [61]. In Germany, especially after OR, the patient is usually relocated to an ICU or IMC. This recommendation stems from an expert consensus and, given a total morbidity of 31.2% for repair of intact AAA in the DGG register in 2015 [62], as well as taking different healthcare systems into account, seems to be justified.
8.2. Techniques
Recommendation.
To avoid incisional hernias, the transverse instead of vertical approach may be considered for OR. Level of evidence 3b/recommendation grade 0.
Recommendation.
The choice of surgical approach (transperitoneal or retroperitoneal) should be left to the surgeon. For inflammatory aneurysms, patients with horseshoe kidneys, and aneurysms with abdominal adhesions, a retroperitoneal approach is strongly recommended. Level of evidence 4/recommendation grade A.
Reason.
It is unclear whether a transperitoneal or retroperitoneal approach is preferable for elective open AAA repair. In a Cochrane review by Ma et al. [63] a total of only 129 participants in 4 randomized trials could be evaluated. With respect to mortality, very low-quality evidence indicated that the retroperitoneal approach has no advantage over the transperitoneal approach. The retroperitoneal approach may increase the risk of postoperative wound infection. Low quality evidence has shown that retroperitoneal access is associated with reduced blood loss, as well as shorter hospital and ICU stays, compared to transperitoneal access. Very low quality evidence revealed no differences between the two approaches for aortic clamping and operation time.
Recommendation.
Prophylactic sublay mesh reinforcement after open AAA repair via midline laparotomy is safe and effectively prevents the high prevalence of incisional herniation after surgery. This technique should be employed. Level of evidence 1b/recommendation grade B.
Reason.
There were two randomized studies that addressed whether the incidence of incisional herniation after AAA open repair via midline laparotomy is reduced by prophylactic sublay mesh reinforcement. A study by Bevis et al. [64] included a total of 85 patients averaging 609 days follow-up in the control group and 939 days in the mesh group. During this time, mesh reinforcement significantly reduced the rate of incisional hernias without increasing the complication rate. A second randomized study included 58 patients in the control group and 56 in the treatment group [65]. Significant differences in postoperative complications between the two groups were not found, apart from pulmonary complications, which were significantly higher in the non-mesh (26%) than in the mesh group (9%). The cumulative incidence of incisional hernias was 17% at 1 year in the control group, compared to 0% in the mesh group. The difference was even clearer after 2 years (28% vs. 0%, respectively). Late complications from the mesh did not arise. In the randomized PRIMA study [66] 480 patients with a midline laparotomy were enrolled, 150 with open AAA repair and 330 with a BMI > 27 kg/m2. The midline laparotomy was either primarily closed at the end of surgery or additionally reinforced with an onlay or sublay mesh. After 2 years follow-up in the group with AAA open repair, the incidence of incisional hernia with onlay mesh was 10/61 (16%) and 10/52 (19%) with sublay mesh, compared to 16/37 (43%) with primary suture. The mesh reinforcement, therefore, significantly reduced the rate of postoperative incisional hernia, as was apparent in the other groups of this study.
Recommendation.
It is strongly recommended that handling characteristics and the surgeon’s preference determine the choice of vascular prosthetic material. Level of evidence 2a/recommendation grade A.
Reason.
Various vascular prostheses are available for aortic replacement, showing no significant differences in terms of long-term patency rates or complications. Pertinent studies on this topic are older and new studies have not been performed [67, 68].
Recommendation.
Should lack of perfusion in the left-sided colon be suspected, it is strongly recommended to re-implant a patent inferior mesenteric artery during OR. Level of evidence 3b/recommendation grade A.
Recommendation.
It is strongly recommended that blood flow to at least one internal iliac artery be maintained during OR. Level of evidence 3b/recommendation grade A.
Reason.
Employing colonoscopy Bast et al. [69] prospectively examined the incidence of ischemic lesions in the rectum and left colon after open AAA reconstruction. The incidence of ischemic bowel lesions of varying severity was 4.5% after iAAA repair and 17.6% after rAAA repair. In this study one patient with intestinal gangrene was registered, making this a rare but here fatal event. Differences in the incidence of postoperative ischemic bowel lesions, depending on whether the inferior mesenteric artery was ligated, was already occluded at surgery, or re-implanted at surgery (4% of cases), were not found. In patients with bilateral occlusion of the internal iliac artery, ischemic bowel lesions were seen in 4 of 11 (36%) cases. According to these findings it is undecided as to whether a patent inferior mesenteric artery should routinely be re-implanted during aortic reconstruction. More importantly, bilateral occlusion of the internal iliac artery should be avoided. Based on a literature review Chitragari et al. [70] described the consequences of an internal iliac artery ligature in various situations. Consequences are most severe in vascular patients. Buttock claudication was reported in 21.2% of cases, buttock necrosis in 5.0%, erectile dysfunction in 2.7%, colon ischemia in 7.0% and spinal cord ischemia in 9.0%.
Recommendation.
Evidence-based intraoperative drug protection to postoperatively improve renal function has not been established. It is strongly recommended to strive for short renal ischemia time and maintenance of the left renal vein during open AAA repair. Level of evidence 4/recommendation grade A.
Reason.
Minimizing renal ischemia time, placing the aortic clamp as far as possible below the renal arterial outlets, avoiding further renal procedures and transection of the left renal vein are among the measures clearly minimizing the risk of postoperative renal dysfunction [71].
Recommendation.
To expedite secondary care it is strongly recommended that acetylsalicylic acid (ASA) be perioperatively continued in AAA patients. Discontinuation should only take place based on individual risk assessment. Level of evidence 3a/recommendation grade A.
Reason.
Optimal timing of surgery for patients on platelet aggregation inhibitors requires interdisciplinary risk stratification with individual risk assessment of perioperative ischemia (stent thrombosis, perioperative myocardial infarction) and bleeding complications. The European Society of Cardiology (ESC) generally recommends in its guidelines [116] that ASA should only be perioperatively discontinued if the bleeding risk during the intervention outweighs potential cardiac benefit. Discontinuation of ASA treatment in previously treated patients should, therefore, only be considered where difficulty with intraoperative hemostasis is expected. For patients receiving dual antiplatelet treatment, it is recommended that elective procedures be postponed until dual therapy has been discontinued and replaced with monotherapy (mostly with ASA).
9. Periprocedural measures and techniques with iAAA—EVAR
9.1 Periprocedural management
GCP: EVAR can be performed under local anesthesia, regional anesthesia, or general anesthesia. There is no clear evidence that one procedure is superior to another in terms of morbidity or mortality.
Assertion.
Radiation exposure during EVAR depends on the equipment, active and passive radiation protection, the volume or body weight of the patient, as well as the training level and expertise of the interventionalist or surgeon. In Germany doctors performing EVAR under X‑ray control must be in possession of the required expertise in radiation protection. Level of evidence 3a.
Recommendation.
It is strongly recommended to administer a single periinterventional dose of cephalosporin for antibiotic prophylaxis in EVAR patients. Level of evidence 5/recommendation grade A.
Reason.
Although no studies concerning periinterventional antibiotic prophylaxis for EVAR exist, prophylaxis is routinely recommended. Even though the risk of infection is less than 1%, if infection does occur, it is a severe complication. In addition, the infection risk increases if additional coil embolization is necessary or endoleaks require treatment.
Recommendation.
It is strongly recommended to hydrate all EVAR patients before, during, and after the procedure, especially those with a GFR < 40 ml/min/1.73 m2. Level of evidence 1b/recommendation grade A.
Reason.
To prevent contrast-induced nephropathy, preinterventional intravenous hydration is recommended for EVAR patients [72]. The ESC guidelines accordingly recommend that all patients, especially those with a GFR < 40 ml/min/1.73 m2, receive 0.9% saline at 1–1.5 ml/kg/h, 12 h prior to angiography and up to 24 h after the procedure [31, 32].
9.2 Techniques
Recommendation.
Anatomical complexity can be preinterventionally classified for EVAR patients using the anatomic severity grading score (ASG score). Evidence level 4/recommendation grade 0.
Reason.
The feasibility of EVAR is limited by anatomic specifics. Anatomic complexity can be classified using the ASG score, as proposed by the Society for Vascular Surgery (SVS)/American Association for Vascular Surgery (AAVS) [73]. The ASG score is generated from a preoperative CT. It correlates with intraoperative difficulty, postprocedural complications, and resource use [74, 75].
Assertion.
Manufacturers’ instructions for prosthesis use should generally be observed; however, an unfavorable anatomy making it impossible to follow the instructions for use does not exclude stent graft implantation. Level of evidence 4.
Recommendation.
Depending on the degree of calcification of the access vessel and the surgeon’s preference, percutaneous access should be chosen whenever possible with EVAR. Percutaneous access is advantageous over open access in terms of intervention time and inguinal infection rate. Level of evidence 2b/recommendation grade B.
Reason.
A systematic review, including observational studies, indicated that the benefits of percutaneous access compared to open access include a significantly lower inguinal infection rate, fewer lymphoceles, a shorter intervention time and inpatient length of stay, with the same level of safety [76]. A Cochrane review on the issue of surgical “cut down” or percutaneous access for bifemoral endograft EVAR ws compiled by Gimzewska et al. [77]. Based on 2 randomized trials with a total of 181 participants, the authors found no differences between the 2 techniques in terms of mortality, major complications or wound infections (moderate quality of evidence). High-quality evidence indicated no differences between the two techniques in bleeding complications and hematoma formation. A moderate quality of evidence supported the view that the percutaneous approach is faster than the surgical approach.
Assertion.
For EVAR treatment of short-necked AAA, if the open procedure is not chosen, fenestrated/branched prostheses and if this is not anatomically feasible, the chimney technique may be employed. Comparatively less published data are available for the chimney technique. Level of evidence 3a.
Recommendation.
Small accessory renal arteries may, if necessary, be overstented at EVAR with relatively little risk. Level of evidence 2c/recommendation grade 0.
Reason.
A literature review including 5 reports with a total of 116 patients established the safety of overstenting accessory renal arteries, if necessary [78]. Segmental renal infarction was reported after overstenting in 0–84% of patients; however, this did not result in impaired renal function, worsening of hypertension, or need for dialysis. This also held true for patients with impaired preinterventional renal function (GFR < 60 ml/h/m2). The risk of a type II endoleak due to an overstented accessory renal artery is negligible. Malgor et al. [79], who also saw no adverse functional effects after overstenting accessory renal arteries, suggested that to avoid type II endoleaks the preinterventional embolization of higher caliber accessory renal arteries (>3 mm) should be carried out if the accessory renal arteries originate from the aneurysmal sac.
Recommendation.
Compared to unilateral or bilateral occlusion of the internal iliac artery, EVAR techniques to preserve the internal iliac artery, mostly by using a branched prosthesis, represent a significant improvement in the treatment of aorto-iliac aneurysms. Techniques to preserve the internal iliac artery are strongly preferred. Level of evidence 2c/recommendation grade A.
Reason.
In the absence of an adequate iliac landing zone, EVAR requires eliminating one or both internal iliac arteries and extending the stent graft into the external iliac artery. To what extent blood flow to the internal iliac artery can be interrupted on one or both sides by embolization or coiling is controversial. Kouvelos et al. [80] found 57 pertinent observational studies. The pooled rate of buttock claudication 30 days after the procedure indicated that 36.5% of patients were affected after bilateral and 27.2% after unilateral interruption of the internal iliac artery. In long-term follow-up, buttock claudication was present in 20.5% of the patients. Other negative consequences of internal iliac artery interruption were buttock necrosis (0.7%), colon ischemia (0.5%), paraplegia (0.3%), and erectile dysfunction (12.7%). In comparison, techniques with iliac preservation (in 87.6% of cases using a branched iliac prosthesis) were associated with fewer complications. An occlusion rate of 5.2% for the internal and 1.7% for the external iliac artery exit points at follow-up, and a buttock claudication of 4.1% were reported.
Recommendation.
Prior to endovascular repair, embolization of an inferior mesenteric artery and/or lumbar arteries may be considered to reduce the endoleak rate. Level of evidence 4/recommendation grade 0.
Reason.
The most common causes of type II endoleaks after EVAR include patent lumbar arteries or reflux from the inferior mesenteric artery, which are usually covered with the endograft. Ward et al. [81] demonstrated on 108 patients that preinterventional coil embolization of the inferior mesenteric artery leads to a significantly lower rate of type II endoleaks, reinterventions, and reduced aneurysm sac size, compared to a control group with a preinterventional patent mesenteric artery. The procedure was not without risk and one patient died due to mesenteric ischemia after coil embolization. To avoid this complication only the inferior mesenteric artery trunk should be embolized, leaving the left colic and superior rectal arteries patent. Significant reduction in type II endoleak rates from preinterventional embolization of the inferior mesenteric artery has also been reported by others [82, 83].
9.3 Postprocedural surveillance
Recommendation.
Extent of intervention, choice of anesthesia, and individual patient risk should determine postinterventional surveillance. Uncomplicated EVAR procedures do not generally require postinterventional intensive care. Level of evidence 4/recommendation grade B.
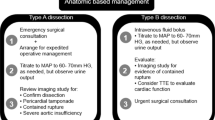
10. Treatment of the rAAA
Assertion.
The rAAA is defined by the clear detection of blood or contrast agent outside the aortic wall. It may, for example, be discovered by preprocedural CT, intraoperative angiography, or during repair.
Recommendation.
It is strongly recommended that patients with confirmed AAA rupture receive immediate invasive treatment. Level of evidence 2b/recommendation grade A.
Reason.
If a patient presents with known AAA, shock and clinical signs of rupture, further diagnostics are not required, and the patient should be immediately transported to the operating room. An emergency ultrasound examination is sufficient to confirm the diagnosis; however, most patients are stable enough to allow CT for further evaluation. A study by Lloyd et al. [84] showed that only 12.5% of patients with rAAA died within 2 h of hospitalization, usually leaving ample time for a CT examination and to consider endovascular intervention (the median interval between hospitalization and death in this retrospective investigation was 10 h and 45 min).
Recommendation.
Depending on the anatomy of the aneurysm and the experience of the surgeon, EVAR is preferable for rAAA repair if OR and EVAR are equally feasible. Level of evidence 1b/recommendation grade B.
Reason.
Several meta-analyses and systematic reviews based on three randomized studies are available on whether OR or EVAR should be given preference for rAAA repair. Sweeting et al. [85] concluded that there is no early survival benefit for endovascular strategy/EVAR for rAAA repair, although very weak evidence favors EVAR after 90 days in patients eligible for OR or EVAR. The meta-analysis indicated that women receiving endovascular strategy/EVAR benefit in terms of early survival, and these patients have a shorter hospital stay. Although the data may be sufficient to favor EVAR, the authors are of the opinion that longer-term data from the three randomized trials are needed for final decision-making.
A Cochrane review [86] based on 4 randomized trials involving 868 participants, was more restrained. No resolution was reached to determine whether EVAR or OR for rAAA repair outperformed the other in terms of 30-day mortality. No robust conclusions could be drawn regarding the 30-day complication rate, although EVAR might have been associated with a lower intestinal ischemia rate than OR. With respect to the results after 6 months and 1 year, only 1 study provided a reliable evaluation and this study reported no clear difference between EVAR and OR. Further randomized studies were encouraged to determine whether differences in outcome between the two approaches are relevant; however, implementation in the future could be difficult for ethical reasons. It has been shown in non-randomized studies that EVAR reduces hospital mortality compared to OR in selected patients suitable for the endovascular procedure. Consequently, if anatomically possible, EVAR is preferable to OR. Since a meta-analysis by van Beek et al. [87] showed that EVAR for rAAA repair is not inferior to OR in terms of short-term survival, it can therefore be recommended to use the same accustomed technique for rAAA as for the intact AAA. In the long term this means a further increase in EVAR for rAAA, especially since an additional literature meta-analysis including large retrospective multicenter studies, clearly favored the endovascular approach for rAAA with respect to in-hospital mortality [88].
Recommendation.
If possible it is strongly recommended that patients with rAAA be treated in specialized, high quality centers; however, variance in geographic conditions influencing transport time, and in local practice and expertise, make it impossible to universally call for the transfer of rAAA patients to centers of excellence. Level of evidence 2a/recommendation grade A.
Reason.
Karthikesalingam et al. [28] showed lower hospital mortality for rAAA patients in both England and the USA treated in academic teaching hospitals, as opposed to non-teaching hospitals. In addition, hospitals with the highest bed capacity in both England and the USA demonstrated lower hospital mortality, compared to smaller hospitals. Further investigation of the relationship between center quality and outcome in treating rAAA was reported by Ozdemir et al. [89] using the English Hospital Episode Statistics database. Hospitals with the lowest mortality rates had significantly more available resources (doctors/bed, nursing staff/bed, intensive care beds). A third study compared short-term and long-term mortality after rAAA treatment in England and Sweden [90]. For both healthcare systems the best results were obtained when patients were treated in hospitals with the highest annual caseload and access to EVAR. These data provide evidence that rAAA patients should be treated in specialized or certified centers with high structural quality.
Recommendation.
Use of an aortic occlusion balloon in EVAR should be considered in patients with hypovolemic shock. Level of evidence 4/recommendation grade B.
Reason.
According to a meta-analysis including 39 studies (1277 patients), a supraceliac aortic occlusion balloon improves EVAR results in hemodynamically unstable patients with rAAA [91].
Recommendation.
Permissive hypotension, aiming to maintain a systolic blood pressure of 80 mm Hg, restricts volume overload and appears sufficient to ensure critical end organ perfusion. Implementation is strongly recommended in patients with rAAA. Level of evidence 3b/recommendation grade A.
Reason.
The permissive hypotension concept aims to reduce the fluid supply in rAAA patients with shock, since massive fluid delivery leads to hemodilution, adversely affecting oxygenation, blood clotting, bleeding due to increased pressure, and thrombus detachment. The aim of preprocedural shock treatment is to provide only enough fluids to maintain a conscious patient, prevent ST changes in the ECG, and maintain the systolic blood pressure at 70–80 mm Hg [42] until surgical control of the bleeding is assured; however, the concept of preprocedural permissive hypotension in patients with rAAA (a patient clientele with high cardiovascular morbidity) is not clearly established. No randomized trials pertaining to this concept could be found in a Cochrane review, leaving the question open as to benefits for rAAA patients [92]. Other investigators [93] also pointed out that although the concept is well-established in animal models, these were young healthy experimental animals having little in common with comorbid elderly rAAA patients; however, it may be considered certain that avoiding volume overload in these patients is beneficial.
GCP: Anesthesia in rAAA patients should be induced in the operating room and only after scrubbing is complete, sterile draping has been placed and a surgeon is ready for surgery at the operating table. Invasive blood pressure monitoring should be initiated before induction of anesthesia but must not delay the onset of surgery. Central venous access should only be established after induction of anesthesia and parallel to the start of the surgical procedure [94].
Recommendation.
When treating rAAA with EVAR, local anesthesia should be given preference over general anesthesia. Level of evidence 3b/recommendation grade B.
Reason.
In the IMPROVE trial [95] patients undergoing EVAR with local anesthesia had a significantly lower mortality even after risk adjustment, than those receiving general anesthesia.
GCP: Following initial care in the (hybrid) operating room, all rAAA patients are admitted to the intensive care unit for further treatment. Patients are at high risk for cardiac, respiratory, and renal complications as well as an abdominal compartment syndrome. Spinal ischemia is a rare, but serious complication after complex aortic intervention. Neuromonitoring should be employed for early identification of a spinal circulatory disorder [94]. An early diagnosis can be hampered by delayed extubation.
Recommendation.
In all rAAA patients at risk, postoperative intra-abdominal pressure (IAP) should be determined via the urinary bladder for early detection of abdominal compartment syndrome (ACS). For persistent IAP > 20 mm Hg and/or IAP > 30 mm Hg, abdominal decompression is indicated. Level of evidence 3b/recommendation grade B.
Reason.
Intra-abdominal hypertension and ACS are considered major risk factors for development of multiple organ failure after OR of rAAA [96]. An international team has defined ACS as present [97, 98] if sustained IAP > 20 mm Hg (with or without abdominal perfusion pressure < 60 mm Hg) is observed, associated with additive organ dysfunction/organ failure. The abdominal perfusion pressure is defined as the mean arterial pressure minus IAP. “Sustained”, means that the IAP measurement must be repeated at least once. “Additive organ dysfunction/organ failure” involves a worsening of vital organ function over time. An ACS can occur after OR, as well as after endovascular rAAA repair [99].
Recommendation.
Vacuum-assisted temporary abdominal closure combined with mesh-mediated fascial traction is preferable in patients with an open abdomen. Level of evidence 4/recommendation grade B.
Reason.
In patients with abdominal hypertension and ACS, early use of the open abdomen significantly improves survival [100]. Various methods for temporary closure of the open abdomen after AAA repair have been analyzed in a systematic literature review [101]. Only seven non-randomized observational studies with small case numbers were available for evaluation and did not allow a definitive conclusion. The vacuum-assisted wound closure with the help of mesh-mediated fascial traction was favored. The subsequent fascial closure after temporary coverage of the open abdomen was 79–100%, the time to closure of the open abdomen averaged 10.5–17 days.
11. Long-term complications
11.1 Complications after OR
Recommendation.
If possible, it is strongly recommended to endovascularly treat para-anastomotic aneurysms after OR. Level of evidence 4/recommendation grade A.
Reason.
In a large series of 58 patients with 80 para-anastomotic aneurysms Ten Bosch et al. [102] found hospital mortality after endovascular revision to be 6.9%, procedural mortality was 10% at 41-months follow-up, and the annual risk of reintervention was 5.8%.
Recommendation.
It is strongly recommended that prosthesis excision and aorto-iliac in situ reconstruction with autologous vein or cryopreserved homografts are the preferred methods of choice for aortic prosthesis infection. Level of evidence 3a/recommendation grade A.
Reason.
Only case series are available to evaluate the treatment of vascular prosthesis infection, and treatment is not standardized. A systematic review with meta-analysis included 12 extra-anatomical bypass studies, 5 studies concerning implanted rifampicin-bound prostheses, 16 studies concerning implanted cryopreserved allografts, and 9 studies concerning high-quality autologous vein reconstruction [103]. Prosthesis excision and autologous vein reconstruction were marked with the lowest pooled reinfection rate and long-term mortality, closely followed by reconstruction with cryopreserved allografts. Combined, and considering all outcome parameters, the ranking favored any in situ option over an extra-anatomic bypass.
Recommendation.
A two-step primary endovascular treatment and subsequent prosthesis excision with in situ revascularization is strongly recommended for aortoenteric fistulas, especially when bleeding occurs. Level of evidence 3b/recommendation grade A.
Reason.
The treatment of aortoenteric fistulas does not principally differ from that of a prosthesis infection. In a systematic literature review with meta-analysis based on 823 patients Kakkos et al. [104] concluded that the endovascular approach is marked with significantly lower clinical mortality than the open approach but in the long term shows a higher sepsis rate and mortality. The survival advantage after 2 years was 51% (endovascular) vs. 40% (open) and, therefore, the difference between EVAR and OR mortality was no longer as evident. They recommended that aortoenteric fistulas should initially be treated by endovascular repair if bleeding is evident and, in a later step, the prosthesis should be excised with in situ revascularization.
Recommendation.
Prior to the procedure it is strongly recommended to inform the patient about a possible worsening of sexual function subsequent to both OR or EVAR. Level of evidence 2b/recommendation grade A.
Reason.
A Swedish study indicated that patients were largely unaware of the negative impact of surgery on sexual function, especially with EVAR. In this group, approximately 80% of patients showed a preoperative, more or less pronounced degree of sexual dysfunction. Both OR and EVAR decreased sexual interest and activity, whereby the sexual function in preoperatively more active men was slightly better after EVAR than after OR during a 1-year follow-up [105].
Assertion.
The incisional hernia is by far the most common long-term complication after OR of the AAA. Disorders of intestinal passage due to adhesions are comparatively rare. Level of evidence 2a.
11.2 Complications after EVAR
Assertion.
A postprocedural acute reversible renal function impairment is expected after both EVAR or OR (more pronounced). In the long term, renal function in these patients deteriorates with age, depending on the initial function. It is not evidence-based that EVAR is at a disadvantage compared to OR due to continued contrast agent administration,. Level of evidence 3a.
Recommendation.
It is strongly recommended to primarily treat endoleaks by interventional EVAR. Level of evidence 4/recommendation grade A.
Recommendation.
It is strongly recommended that type I endoleaks, whose elimination is not interventionally possible, initially be closely monitored in the absence of aneurysm growth. It is strongly recommended that treatment follows in the case of growth. Level of evidence 4/recommendation grade A.
Recommendation.
It is strongly recommended that type II endoleaks without an increase in aneurysm sac diameter should be kept under observation. Level of evidence 3a/recommendation grade A.
Recommendation.
It is strongly recommended to treat type II endoleaks with increasing aneurysm sac diameter. Level of evidence 3a/recommendation grade A.
Recommendation.
It is strongly recommended to treat type III endoleaks. Level of evidence 3a/recommendation grade A.
Recommendation.
It is strongly recommended not to treat type IV endoleaks that are not growing. Level of evidence 3a/recommendation grade A.
Recommendation.
Type V endoleaks (endotension). Enlargement of an AAA after EVAR, without evidence of an endoleak, with an increase in diameter > 10 mm over 12 months or >5 mm in 6 months, should be surgically treated or treated with a new stent graft. Level of evidence 3a/recommendation grade B.
Recommendation.
Stent migration > 10 mm with an increase in aneurysm diameter and/or evidence of an endoleak may require endovascular treatment. Level of evidence 4/recommendation grade 0.
Recommendation.
Iliac femoral occlusion is described in approximately 4% of cases after EVAR and it is strongly recommended to address them endovascularly. If not possible, thrombectomy and femorofemoral crossover bypass are the therapeutic alternatives. Level of evidence 3a/recommendation grade A.
Recommendation.
As a rule for stent graft infections, it is strongly recommended that the infected prosthesis material be removed and revascularization performed. Level of evidence 4/recommendation grade A. In selected cases, however, a conservative approach may be considered. Level of evidence 4/recommendation grade 0.
Reason.
The risk of stent graft infection is low. In a follow-up of >8 years Patel et al. [106] in the EVAR trial 1 rated life-threatening reintervention, which included conversion to an open procedure, recurrent EVAR, and treatment of graft infections, at 2.1/100 person-years. The treatment of stent graft infections is not standardized, with only case series [107] being available. A conservative approach should certainly be considered in patients with a limited life expectancy and high comorbidity, since the treatment of stent graft infections generally involves removing the foreign material and subsequent revascularization [108, 109]. The most severe form of prosthetic infection is the aortoenteric fistula. In an overview by Moulakakis et al. [110], no patient with such an infection survived a conservative treatment approach. The fastest way to stop bleeding in this situation is to cover the fistula with a new endograft [107]; however, this can only be considered a bridging measure.
12 Aftercare/follow-up imaging
12.1. Follow-up after OR
Recommendation.
After OR, patients should undergo CT control at 5‑year intervals. Level of evidence 4/recommendation grade 0.
12.2. Follow-up after EVAR
Recommendation.
After EVAR, ultrasound should be conducted as a standard. Regular postprocedural follow-up should be organized by the vascular center that carried out the implantation. Level of evidence 3/recommendation grade B.
(Note: the proposed aftercare scheme is shown as a flowchart in the extended guidelines).
13. Special issues
13.1. Inflammatory aneurysms
Recommendation.
In patients with inflammatory AAA it is strongly recommended that the indication for OR and EVAR be made according to the same criteria as in non-inflammatory AAA. The use of EVAR has a lower 1‑year mortality but in patients with hydronephrosis and low surgical risk OR may be preferable. Level of evidence 4/recommendation grade A.
Reason.
In a systematic review based on 56 retrospective surveys Paravastu et al. [111] addressed whether inflammatory AAA should be treated by OR or EVAR. The 30-day mortality was 6.2% after OR and 2.4% after EVAR. At follow-up, receding peri-aortic inflammation was seen in 73% after OR and 65% after EVAR. Hydronephrosis regressed 69% after OR and 38% after EVAR. The 1‑year aneurysm-related mortality was 2% (OR) vs. 0% (EVAR). Overall mortality was 14% (OR) vs. 2% (EVAR). Another analysis concerning inflammatory AAA concluded that EVAR is associated with shorter surgery duration, lower need for transfusion and lower morbidity compared to OR [112]. Nonetheless, there is no evidence-based proof that EVAR or OR should be preferred in inflammatory AAA. A Cochrane review found neither a randomized nor controlled study concerning this issue [113].
13.2. Connective tissue disease (Marfan, Loeys-Dietz syndrome [LDS], Ehlers-Danlos syndrome)
Recommendation.
The incidence of AAA accompanying congenital connective tissue diseases is rare. If an intervention is indicated, open surgical intervention should initially be employed in cases of Marfan syndrome and other hereditary connective tissue disorders. Secondary therapy can be endovascular. Level of evidence 4/recommendation grade B.
13.3 Treatment of the mycotic AAA
Recommendation.
Mycotic AAA should be treated openly or endovascularly, depending on the patient’s condition and the experience of the treatment team. Level of evidence 4/recommendation grade B.
Reason.
The term mycotic AAA (mAAA) refers to an infection (usually bacterial) of the aorta with subsequent aneurysm formation. Based on the Swedish national vascular registry, Sörelius et al. [114] evaluated the largest cohort of mAAA on a long-term basis. After excluding patients with prosthetic infections or previous aortic surgery, they evaluated 132 patients treated between 1994 and 2014. The EVAR procedure was first introduced in 2001 and has since accounted for 59% of all mAAA repairs. Short-term survival of patients was significantly better after EVAR than after OR (after 30 days OR 89%, EVAR 99%; after 3 months OR 74%, EVAR 96%). The trend was still present after 1 year (OR 73%, EVAR 84%); however, there were no differences in 5‑year survival (OR 60%, EVAR 58%) and 10-year survival (OR 39%, EVAR 41%).
Literatur
Chaikof EL, Dalman RL, Eskandari MK et al (2018) The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg 67:2–77
Jahangir E, Lipworth L, Edwards TL, Kabagambe EK, Mumma MT, Mensah GA, Fazio S, Blot WJ, Sampson UK (2015) Smoking, sex, risk factors and abdominal aortic aneurysms: a prospective study of 18 782 persons aged above 65 years in the Southern Community Cohort Study. J Epidemiol Community Health 69:481–488
Tang W, Yao L, Roetker NS, Alonso A, Lutsey PL, Steenson CC, Lederle FA, Hunter DW, Bengtson LG, Guan W, Missov E, Folsom AR (2016) Lifetime risk and risk factors for abdominal aortic aneurysm in a 24-year prospective study: the ARIC Study (Atherosclerosis Risk in Communities). Arterioscler Thromb Vasc Biol 36:2468–2477
Takagi H, Umemoto T, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group (2017) Association of hypertension with abdominal aortic aneurysm expansion. Ann Vasc Surg 39:74–89
Rughani G, Robertson L, Clarke M (2012) Medical treatment for small abdominal aortic aneurysms. Cochrane Database Syst Rev 9:CD9536. https://doi.org/10.1002/14651858.cd009536
Twine CP, Williams IM (2011) Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms. Br J Surg 98:346–353
Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, Verhagen HJ, Khan NA, Dunkelgrun M, Bax JJ, Poldermans D, Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group (2009) Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 361:980–989
Galiñanes EL, Reynolds S, Dombrovskiy VY, Vogel TR (2015) The impact of preoperative statin therapy on open and endovascular abdominal aortic aneurysm repair outcomes. Vascular 23(2015):344–349
Robertson L, Atallah E, Stansby G (2014) Pharmacological treatment of vascular risk factors for reducing mortality and cardiovascular events in patients with abdominal aortic aneurysm. Cochrane Database Syst Rev 1:CD10447. https://doi.org/10.1002/14651858.cd010447.pub2
Erbel R, Aboyans V, Boileau C, ESC Committee for Practice Guidelines (2014) 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J 35(41):2873–2926. https://doi.org/10.1093/eurheartj/ehu281 (The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC))
Bahia SS, Vidal-Diez A, Seshasai SR, Shpitser I, Brownrigg JR, Patterson BO, Ray KK, Holt PJ, Thompson MM, Karthikesalingam A (2016) Cardiovascular risk prevention and all-cause mortality in primary care patients with an abdominal aortic aneurysm. Br J Surg 103:1626–1633
de Bruin JL, Baas AF, Heymans MW, Buimer MG, Prinssen M, Grobbee DE, Blankensteijn JD, DREAM Study Group (2014) Statin therapy is associated with improved survival after endovascular and open aneurysm repair. J Vasc Surg 59:39–44
Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH, Pharmaceutical Aneurysm Stabilisation Trial Study Group (2013) Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med 159:815–823
Fleisher LA, Fleischmann KE, Auerbach AD, American College of Cardiology, American Heart Association (2014) 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. J Am Coll Cardiol 64:e77–e137 (A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines)
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) (2015) Ultraschall-Screening auf Bauchaortenaneurysmen (IQWiG-Berichte – Nr. 294. Abschlussbericht S13-04. ISSN: 1864-2500. Stand: 02.04.2015)
Cosford PA, Leng GC (2007) Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd002945.pub2
Ulug P, Powell JT, Sweeting MJ, Bown MJ, Thompson SG, SWAN Collaborative Group (2016) Meta-analysis of the current prevalence of screen-detected abdominal aortic aneurysm in women. Br J Surg 103:1097–1104
Lederle FA, Johnson GR, Wilson SE, Chute EP, Hye RJ, Makaroun MS, Barone GW, Bandyk D, Moneta GL, Makhoul RG (2000) The aneurysm detection and management study screening program: validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch Intern Med 160:1425–1430
Larsson E, Granath F, Swedenborg J, Hultgren R (2009) A population-based case-control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg 2009(49):47–50
RESCAN Collaborators, Bown MJ, Sweeting MJ, Brown LC, Powell JT, Thompson SG (2013) Surveillance intervals for small abdominal aortic aneurysms: a meta-analysis. JAMA 309:806–813
Thompson SG, Brown LC, Sweeting MJ, Bown MJ, Kim LG, Glover MJ, Buxton MJ, Powell JT (2013) Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: implications for surveillance intervals and their cost-effectiveness. Health Technol Assess 17:1–118
Schäberle W, Leyerer L, Schierling W, Pfister K (2014) Ultraschalldiagnostik der abdominellen Aorta. Gefasschirurgie 19:558–563
Filardo G, Powell JT, Martinez MA, Ballard DJ (2015) Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev 2:CD1835. https://doi.org/10.1002/14651858.cd001835.pub4
United Kingdom Small Aneurysm Trial Participants (2002) Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med 346:1445–1452
Brown PM, Zelt DT, Sobolev B (2003) The risk of rupture in untreated aneurysms: the impact of size, gender, and expansion rate. J Vasc Surg 37:280–284
De Martino RR, Nolan BW, Goodney PP, Chang CK, Schanzer A, Cambria R, Bertges DJ, Cronenwett JL, Vascular Study Group of Northern New England (2010) Outcomes of symptomatic abdominal aortic aneurysm repair. J Vasc Surg 52:5–12
Soden PA, Zettervall SL, Ultee KH, Darling JD, Buck DB, Hile CN, Hamdan AD, Schermerhorn ML (2016) Outcomes for symptomatic abdominal aortic aneurysms in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg 64:297–305
Karthikesalingam A, Holt PJ, Vidal-Diez A, Ozdemir BA, Poloniecki JD, Hinchliffe RJ, Thompson MM (2014) Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet 383(9921):963–969
Khashram M, Williman JA, Hider PN, Jones GT, Roake JA (2016) Systematic review and meta-analysis of factors influencing survival following abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 51:203–215
Galle J (2016) Klug Entscheiden … in der Nephrologie. Dtsch Arztebl 113(35-36):A 1534–A 1537
Rear R, Bell RM, Hausenloy DJ (2016) Contrast-induced nephropathy following angiography and cardiac interventions. Heart 102:638–648
Windecker S, Kolh P, Alfonso F et al (2014) 2014 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 35:2541–2619 (The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI))
Thomsen HS, Webb JAW (eds) (2014) Contrast media. Safety issues and ESUR guidelines, 3 edn. Springer, Berlin, Heidelberg
Kurvers HA, van der Graaf Y, Blankensteijn JD, Visseren FL, Eikelboom B, SMART Study Group (2003) Screening for asymptomatic internal carotid artery stenosis and aneurysm of the abdominal aorta: comparing the yield between patients with manifest atherosclerosis and patients with risk factors for atherosclerosis only. J Vasc Surg 37:1226–1233
Vranes M, Davidovic L, Vasic D, Radmili O (2013) Coexistence of internal carotid artery stenosis in patients with abdominal aortic aneurysm. Korean Circ J 43:550–556
Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (DGAI), Deutsche Gesellschaft für Innere Medizin (DGIM), Deutsche Gesellschaft für Chirurgie (DGCH), Zwissler B (2017) Präoperative Evaluation erwachsener Patienten vor elektiven, nicht Herz-Thoraxchirurgischen Eingriffen. Anaesthesist 66:442–458
Grant SW, Hickey GL, Grayson AD, Mitchell DC, McCollum CN (2013) National risk prediction model for elective abdominal aortic aneurysm repair. Br J Surg 100:645–653
Grant SW, Hickey GL, Carlson ED, McCollum CN (2014) Comparison of three contemporary risk scores for mortality following elective abdominal aortic aneurysm repair. Eur J Vasc Endovasc Surg 48:38–44
Corriere MA, Islam A, Craven TE, Conlee TD, Hurie JB, Edwards MS (2014) Influence of computed tomography angiography reconstruction software on anatomic measurements and endograft component selection for endovascular abdominal aortic aneurysm repair. J Vasc Surg 59:1224–1231
Biancari F, Paone R, Venermo M, D’Andrea V, Perälä J (2013) Diagnostic accuracy of computed tomography in patients with suspected abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg 45:227–230
Diwan A, Sarkar R, Stanley JC, Zelenock GB, Wakefield TW (2000) Incidence of femoral and popliteal artery aneurysms in patients with abdominal aortic aneurysms. J Vasc Surg 31:863–869
Pereira BM, Chiara O, Ramponi F et al (2015) WSES position paper on vascular emergency surgery. World J Emerg Surg 10:49
Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD (2013) Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg 100:863–872
Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM (2014) Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.cd004178.pub2
McCutcheon BA, Talamini MA, Chang DC, Rose JA, Bandyk DF, Wilson SE (2013) The comparative effectiveness of surgeons over interventionalists in endovascular repairs of abdominal aortic aneurysm. Ann Surg 258:476–482
Marlow NE, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC, Maddern GJ (2010) Effect of hospital and surgeon volume on patient outcomes following treatment of abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg 40:572–579
Hicks CW, Wick EC, Canner JK, Black JH 3rd, Arhuidese I, Qazi U, Obeid T, Freischlag JA, Malas MB (2015) Hospital-level factors associated with mortality after endovascular and open abdominal aortic aneurysm repair. Jama Surg 150:632–636
Phillips P, Poku E, Essat M, Woods HB, Goka EA, Kaltenthaler EC, Walters S, Shackley P, Michaels J (2017) Procedure volume and the association with short-term mortality following abdominal aortic aneurysm repair in European populations: a systematic review. Eur J Vasc Endovasc Surg 53:77–88
Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM (2007) Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg 94:395–403
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL (2003) Surgeon volume and operative mortality in the United States. N Engl J Med 349(22):2117–2127. https://doi.org/10.1056/nejmsa035205
Kasirajan V, Hertzer NR, Beven EG, O’Hara PJ, Krajewski LP, Sullivan TM (1998) Management of isolated common iliac artery aneurysms. Cardiovasc Surg 6:171–177
Santilli SM, Wernsing SE, Lee ES (2000) Expansion rates and outcomes for iliac artery aneurysms. J Vasc Surg 31(1 Pt 1):114–121
Huang Y, Gloviczki P, Duncan AA, Kalra M, Hoskin TL, Oderich GS, McKusick MA, Bower TC (2008) Common iliac artery aneurysm: Expansionrate and results of open surgical and endovascular repair. J Vasc Surg 47:1203–1211
Stewart A, Eyers PS, Earnshaw JJ (2006) Prevention of infection in arterial reconstruction. Cochrane Database Syst Rev 3:CD3073. https://doi.org/10.1002/14651858.cd003073.pub2
Torossian A, Bein B, Bräuer A et al (2014) S3 Leitlinie Vermeidung von perioperativer Hypothermie (AWMF-Register Nr. 001/018. Klasse: S3)
Meybohm P, Schmitz-Rixen T, Steinbicker A, Schwenk W, Zacharowski K (2017) Das Patient-Blood-Management-Konzept. Chirurg 88:867–870 (Gemeinsame Empfehlung der Deutschen Gesellschaft für Anästhesiologie und Intensivmedizin und der Deutschen Gesellschaft für Chirurgie)
Waurick K (2015) Antikoagulantien und Regionalanästhesie – wie verfahren? (Refresher Course Nr. 41. Aktuelles Wissen für Anästhesisten. Herausgegeben von der Deutschen Akademie für Anästhesiologische Fortbildung)
Pöpping DM, Elia N, Van Aken HK, Marret E, Schug SA, Kranke P, Wenk M, Tramèr MR (2014) Impact of epidural analgesia on mortality and morbidity after surgery: systematic review and meta-analysis of randomized controlled trials. Ann Surg 259:1056–1067
Guay J, Kopp S (2016) Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev 1:CD5059. https://doi.org/10.1002/14651858.cd005059.pub4
Carl M, Alms A, Braun J et al (2010) S3-Leitlinie zur intensivmedizinischen Versorgung herzchirurgischer Patienten – Hämodynamisches Monitoring und Herz-Kreislauf-Therapie (AWMF Register 001/016)
Deutsche Gesellschaft für Anästhesiologie und Intensivmedizin (2014) S3-Leitlinie Intravasale Volumentherapie beim Erwachsenen (AWMF-Registernummer 001/020)
Schmitz-Rixen T, Steffen M, Grundmann RT (2017) Versorgung des abdominellen Aortenaneurysmas (AAA) 2015. Registerbericht des DIGG der DGG. Gefasschirurgie 22:180–188
Ma B, Wang YN, Chen KY, Zhang Y, Pan H, Yang K (2016) Transperitoneal versus retroperitoneal approach for elective open abdominal aortic aneurysm repair. Cochrane Database Syst Rev 2:CD10373. https://doi.org/10.1002/14651858.cd010373.pub2
Bevis PM, Windhaber RA, Lear PA, Poskitt KR, Earnshaw JJ, Mitchell DC (2010) Randomized clinical trial of mesh versus sutured wound closure after open abdominal aortic aneurysm surgery. Br J Surg 97:1497–1502
Muysoms FE, Detry O, Vierendeels T, Huyghe M, Miserez M, Ruppert M, Tollens T, Defraigne JO, Berrevoet F (2016) Prevention of incisional hernias by prophylactic mesh-augmented reinforcement of midline laparotomies for abdominal aortic aneurysm treatment: a randomized controlled trial. Ann Surg 263:638–645
Jairam AP, Timmermans L, Eker HH, PRIMA Trialist Group (2017) Prevention of incisional hernia with prophylactic onlay and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2‑year follow-up of a multicentre, double-blind, randomised controlled trial. Lancet 390(10094):567–576
Polterauer P, Prager M, Hölzenbein T, Karner J, Kretschmer G, Schemper M (1992) Dacron versus polytetrafluoroethylene for Y‑aortic bifurcation grafts: a six-year prospective, randomized trial. Surgery 111:626–633
Prager MR, Hoblaj T, Nanobashvili J, Sporn E, Polterauer P, Wagner O, Böhmig HJ, Teufelsbauer H, Ploner M, Huk I (2003) Collagen- versus gelatine-coated Dacron versus stretch PTFE bifurcation grafts for aortoiliac occlusive disease: long-term results of a prospective, randomized multicenter trial. Surgery 134:80–85
Bast TJ, van der Biezen JJ, Scherpenisse J, Eikelboom BC (1990) Ischaemic disease of the colon and rectum after surgery for abdominal aortic aneurysm: a prospective study of the incidence and risk factors. Eur J Vasc Surg 4:253–257
Chitragari G, Schlosser FJ, Ochoa Chaar CI, Sumpio BE (2015) Consequences of hypogastric artery ligation, embolization, or coverage. J Vasc Surg 62(5):1340–1347. https://doi.org/10.1016/j.jvs.2015.08.053
Dubois L, Durant C, Harrington DM, Forbes TL, Derose G, Harris JR (2013) Technical factors are strongest predictors of postoperative renal dysfunction after open transperitoneal juxtarenal abdominal aortic aneurysm repair. J Vasc Surg 57:648–654
Kawatani Y, Nakamura Y, Mochida Y et al (2016) Contrast medium induced nephropathy after endovascular stent graft placement: An examination of its prevalence and risk factors. Radiol Res Pract 2016:1–5. https://doi.org/10.1155/2016/5950986
Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, Bernhard VM, Harris PL, Kent KC, May J, Veith FJ, Zarins CK (2002) Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg 35:1061–1066
Ahanchi SS, Carroll M, Almaroof B, Panneton JM (2011) Anatomic severity grading score predicts technical difficulty, early outcomes, and hospital resource utilization of endovascular aortic aneurysm repair. J Vasc Surg 54:1266–1272
Rasheed K, Cullen JP, Seaman MJ, Messing S, Ellis JL, Glocker RJ, Doyle AJ, Stoner MC (2016) Aortic anatomic severity grade correlates with resource utilization. J Vasc Surg 63:569–576
Hajibandeh S, Hajibandeh S, Antoniou SA, Child E, Torella F, Antoniou GA (2016) Percutaneous access for endovascular aortic aneurysm repair: A systematic review and meta-analysis. Vascular 24:638–648
Gimzewska M, Jackson AI, Yeoh SE, Clarke M (2017) Totally percutaneous versus surgical cut-down femoral artery access for elective bifurcated abdominal endovascular aneurysm repair. Cochrane Database Syst Rev 2:CD10185. https://doi.org/10.1002/14651858.cd010185.pub3
Antoniou GA, Karkos CD, Antoniou SA, Georgiadis GS (2013) Can an accessory renal artery be safely covered during endovascular aortic aneurysm repair? Interact Cardiovasc Thorac Surg 17:1025–1027
Malgor RD, Oderich GS, Vrtiska TJ, Kalra M, Duncan AA, Gloviczki P, Cha S, Bower TC (2013) A case-control study of intentional occlusion of accessory renal arteries during endovascular aortic aneurysm repair. J Vasc Surg 58:1467–1475
Kouvelos GN, Katsargyris A, Antoniou GA, Oikonomou K, Verhoeven EL (2016) Outcome after interruption or preservation of internal iliac artery flow during endovascular repair of abdominal aorto-iliac aneurysms. Eur J Vasc Endovasc Surg 52:621–634
Ward TJ, Cohen S, Fischman AM, Kim E, Nowakowski FS, Ellozy SH, Faries PL, Marin ML, Lookstein RA (2013) Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol 24:49–55
Manunga JM, Cragg A, Garberich R, Urbach JA, Skeik N, Alexander J, Titus J, Stephenson E, Alden P, Sullivan TM (2017) Preoperative inferior mesenteric artery embolization: a valid method to reduce the rate of type II Endoleak after EVAR? Ann Vasc Surg 39:40–47
Müller-Wille R, Uller W, Gössmann H, Heiss P, Wiggermann P, Dollinger M, Kasprzak P, Pfister K, Stroszczynski C, Wohlgemuth WA (2014) Inferior mesenteric artery embolization before endovascular aortic aneurysm repair using amplatzer vascular plug type 4. Cardiovasc Intervent Radiol 37:928–934
Lloyd GM, Bown MJ, Norwood MG, Deb R, Fishwick G, Bell PR, Sayers RD (2004) Feasibility of preoperative computer tomography in patients with ruptured abdominal aortic aneurysm: a time-to-death study in patients without operation. J Vasc Surg 39:788–791
Sweeting MJ, Balm R, Desgranges P, Ulug P, Powell JT, Ruptured Aneurysm Trialists (2015) Individual-patient meta-analysis of three randomized trials comparing endovascular versus open repair for ruptured abdominal aortic aneurysm. Br J Surg 102:1229–1239
Badger S, Forster R, Blair PH, Ellis P, Kee F, Harkin DW (2017) Endovascular treatment for ruptured abdominal aortic aneurysm. Cochrane Database Syst Rev 5:CD5261. https://doi.org/10.1002/14651858.cd005261.pub4
van Beek SC, Conijn AP, Koelemay MJ, Balm R (2014) Editor’s Choice—Endovascular aneurysm repair versus open repair for patients with a ruptured abdominal aortic aneurysm: a systematic review and meta-analysis of short-term survival. Eur J Vasc Endovasc Surg 47:593–602
Qin C, Chen L, Xiao YB (2014) Emergent endovascular vs. open surgery repair for ruptured abdominal aortic aneurysms: a meta-analysis. PLoS ONE 9(1):e87465. https://doi.org/10.1371/journal.pone.0087465
Ozdemir BA, Karthikesalingam A, Sinha S, Poloniecki JD, Vidal-Diez A, Hinchliffe RJ, Thompson MM, Holt PJ (2015) Association of hospital structures with mortality from ruptured abdominal aortic aneurysm. Br J Surg 102:516–524
Karthikesalingam A, Wanhainen A, Holt PJ, Vidal-Diez A, Brownrigg JR, Shpitser I, Björck M, Thompson MM, Mani K (2016) Comparison of long-term mortality after ruptured abdominal aortic aneurysm in England and Sweden. Br J Surg 103:199–206
Karkos CD, Papadimitriou CT, Chatzivasileiadis TN, Kapsali NS, Kalogirou TE, Giagtzidis IT, Papazoglou KO (2015) The impact of aortic occlusion balloon on mortality after endovascular repair of ruptured abdominal aortic aneurysms: a meta-analysis and meta-regression analysis. Cardiovasc Intervent Radiol 38:1425–1437
Moreno DH, Cacione DG, Baptista-Silva JC (2016) Controlled hypotension versus normotensive resuscitation strategy for people with ruptured abdominal aortic aneurysm. Cochrane Database Syst Rev 5:CD11664. https://doi.org/10.1002/14651858.cd011664.pub2
Roberts K, Revell M, Youssef H, Bradbury AW, Adam DJ (2006) Hypotensive resuscitation in patients with ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 31:339–344
Hope K, Nickols G, Mouton R (2016) Modern anesthetic management of ruptured abdominal aortic aneurysms. J Cardiothorac Vasc Anesth 30:1676–1684
IMPROVE trial investigators, Powell JT, Hinchliffe RJ, Thompson MM et al (2014) Observations from the IMPROVE trial concerning the clinical care of patients with ruptured abdominal aortic aneurysm. Br J Surg 101:216–224
Rasmussen TE, Hallett JW Jr, Noel AA, Jenkins G, Bower TC, Cherry KJ Jr, Panneton JM, Gloviczki P (2002) Early abdominal closure with mesh reduces multiple organ failure after ruptured abdominal aortic aneurysm repair: guidelines from a 10-year case-control study. J Vasc Surg 35:246–253
Björck M, Wanhainen A (2014) Management of abdominal compartment syndrome and the open abdomen. Eur J Vasc Endovasc Surg 47:279–287
Kirkpatrick AW, Roberts DJ, De Waele J, The Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome (2013) Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med 39:1190–1206
Rubenstein C, Bietz G, Davenport DL, Winkler M, Endean ED (2015) Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J Vasc Surg 61:648–654
Cheatham ML, Safcsak K (2010) Is the evolving management of intra-abdominal hypertension and abdominal compartment syndrome improving survival? Crit Care Med 38:402–407
Acosta S, Wanhainen A, Björck M (2016) Temporary abdominal closure after abdominal aortic aneurysm repair: a systematic review of contemporary observational studies. Eur J Vasc Endovasc Surg 51(3):371–378. https://doi.org/10.1016/j.ejvs.2015.10.014
Ten Bosch JA, Waasdorp EJ, de Vries JP, Moll FL, Teijink JA, van Herwaarden JA (2011) The durability of endovascular repair of para-anastomotic aneurysms after previous open aortic reconstruction. J Vasc Surg 54:1571–1578
O’Connor S, Andrew P, Batt M, Becquemin JP (2006) A systematic review and meta-analysis of treatments for aortic graft infection. J Vasc Surg 44:38–45
Kakkos SK, Bicknell CD, Tsolakis IA, Bergqvist D, Hellenic Co-operative Group on Aortic Surgery (2016) Editor’s Choice—Management of secondary aorto-enteric and other abdominal arterio-enteric fistulas: a review and pooled data analysis. Eur J Vasc Endovasc Surg 52:770–786
Pettersson M, Mattsson E, Bergbom I (2009) Prospective follow-up of sexual function after elective repair of abdominal aortic aneurysms using open and endovascular techniques. J Vasc Surg 50:492–499
Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators (2016) Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet 388(10058):2366–2374
Setacci C, Chisci E, Setacci F, Ercolini L, de Donato G, Troisi N, Galzerano G, Michelagnoli S (2014) How to diagnose and manage infected endografts after endovascular aneurysm repair. Aorta (Stamford) 2:255–264
Laser A, Baker N, Rectenwald J, Eliason JL, Criado-Pallares E, Upchurch GR Jr (2011) Graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 54:58–63
Phade SV, Keldahl ML, Morasch MD, Rodriguez HE, Pearce WH, Kibbe MR, Eskandari MK (2011) Late abdominal aortic endograft explants: indications and outcomes. Surgery 150(4):788–795. https://doi.org/10.1016/j.surg.2011.07.061
Moulakakis KG, Sfyroeras GS, Mylonas SN, Mantas G, Papapetrou A, Antonopoulos CN, Kakisis JD, Liapis CD (2014) Outcome after preservation of infected abdominal aortic endografts. J Endovasc Ther 21:448–455
Paravastu SC, Ghosh J, Murray D, Farquharson FG, Serracino-Inglott F, Walker MG (2009) A systematic review of open versus endovascular repair of inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg 38:291–297
Kakkos SK, Papazoglou KO, Tsolakis IA, Lampropoulos G, Papadoulas SI, Antoniadis PN (2015) Open versus endovascular repair of inflammatory abdominal aortic aneurysms: a comparative study and meta-analysis of the literature. Vasc Endovascular Surg 49:110–118
Capoccia L, Riambau V (2015) Endovascular repair versus open repair for inflammatory abdominal aortic aneurysms. Cochrane Database Syst Rev 4:CD10313. https://doi.org/10.1002/14651858.cd010313.pub2
Sörelius K, Wanhainen A, Furebring M, Björck M, Gillgren P, Mani K, Swedish Collaborator Group for Mycotic Abdominal Aortic Aneurysms (2016) Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation 134:1822–1832
Mehta M, Taggert J, Darling RC 3rd, Chang BB, Kreienberg PB, Paty PS, Roddy SP, Sternbach Y, Ozsvath KJ, Shah DM (2006) Establishing a protocol for endovascular treatment of ruptured abdominal aortic aneurysms: outcomes of a prospective analysis. J Vasc Surg 44:1–8
Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery, European Society of Cardiology (ESC), Poldermans D, Bax JJ, Boersma E et al (2009) Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 30:2769–2812
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.S. Debus, F. Heidemann, W. Gross-Fengels, A. Mahlmann, E. Muhl, K. Pfister, S. Roth, C. Stroszczynski, A. Walther, N. Weiss, M. Wilhelmi and R.T. Grundmann declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Additional information
Participating professional societies and organizations
German Society for Vascular Surgery and Vascular Medicine (DGG, E.S. Debus); German Radiological Society (DRG, W. Gross-Fengels); German Society for Angiology/Society for Vascular Medicine (DGA, A. Mahlmann, N. Weiss); German Interdisciplinary Association of Intensive Care and Emergency Medicine (DIVI, E. Muhl); German Association of Ultrasound in Medicine (DEGUM, K. Pfister); German Vascular League e. V. (S. Roth); German Society of Interventional Radiology (DEGIR, Ch. Stroszczynski); German Society of Anesthesiology and Intensive Care Medicine (DGAI, A. Walther); German Society for Thoracic and Cardiovascular Surgery (DGTHG, M. Wilhelmi); German Society of Surgery (DGCH, R.T. Grundmann)
E.S. Debus: steering group speaker, F. Heidemann: steering group secretary, R.T. Grundmann: steering group.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Debus, E.S., Heidemann, F., Gross-Fengels, W. et al. Short version of the S3 guideline on screening, diagnosis, therapy and follow-up of abdominal aortic aneurysms. Gefässchirurgie 24 (Suppl 1), 1–18 (2019). https://doi.org/10.1007/s00772-018-0465-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00772-018-0465-x