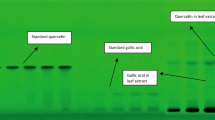
Abstract
The current research aimed to investigate the pharmacognostical, phytochemical and quantification of rutin in leaves of Phoenix sylvestris by high-performance thin-layer chromatographic (HPTLC) method. Various extracts of the leaves of P. sylvestris were prepared in different solvents by cold maceration method. The extracts were subjected to various chemical tests for detection of various secondary metabolites as well as to determine the amount of total phenolic content (TPC) and total flavonoids content (TFC). The methanolic extract was subjected to carry out quantification of rutin by HPTLC method. The method was validated for linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), etc. Pharmacognostic study mainly covered the macroscopic and microscopic features of the leaves including powder microscopy. Qualitative analysis revealed the existence of certain chemical constituents such as flavonoids, tannins, organic acids and saponin glycosides. The methanolic extract of P. sylvestris showed LOD and LOQ for rutin as 125.01 ng/spot and 204.921 ng/spot, respectively. The amount of rutin was detected as 54.6 mg g-1 of extract.

Graphical abstract








Similar content being viewed by others
Abbreviations
- P. sylvestris :
-
Phoenix sylvestris
- TPC:
-
total phenolic content
- TFC:
-
total flavonoid content
- PSLPEE:
-
petroleum ether extract of leaf of Phoenix sylvestris
- PSLCLE:
-
chloroform extract of leaf of Phoenix sylvestris
- PSLME:
-
methanolic extract of leaf of Phoenix sylvestris
- PSLHAE:
-
hydroalcoholic extract of leaf of Phoenix sylvestris
- PSLAE:
-
aqueous extract of leaf of Phoenix sylvestris
- LOD:
-
limit of detection
- LOQ:
-
limit of quantification
- ng:
-
nano-gram
References
Jain P, Jain S, Sharma S, Paliwal S (2018) Diverse application of Phoenix sylvestris: a potential herb. Agric Nat Res 52:107–114
Chakraborty P, Roy I, Chatterjee S, Chanda S, Bhattacharya SG (2006) Phoenix sylvestris roxb pollen allergy: a 2-year randomized controlled trial and follow-up study of immunotherapy in patients with seasonal allergy in an agricultural area of West Bengal. India J Investig Allergol Clin Immunol 16:377–384
Anonymous, Encyclopedia of agricultural science. 4. Anmol publications Pvt. Ltd., New Delhi, 2000, pp. 125–155
Chowdhury MSH, Halim MA, Muhammed N, Haque F, Koike M (2008) Traditional utilization of wild date palm (Phoenix sylvestris) in rural Bangladesh: an approach to sustainable biodiversity management. J Forest Res 19:245–251
Kulkarni AR, Mulani RM (2004) Indigenous palms of India. Curr Sci 86:1598–1603
Rana MP, Islam MS (2010) The role of palm husbandry in the rural economy of the south-eastern region of Bangladesh. IForest 3:39–43
Barrow SC (1998) A monograph of Phoenix L. (Palmae: Coryphoideae). Key Bulletin 53:513–575
Wallis TE (ed) (1985) Textbook of Pharmacognosy, 5th edn. CBS Publishers & Distributors, New Delhi
Brain KR, Turner TD (1975) The practical evaluation of phyto pharmaceuticals, 1st edn. Wright-Scientechnica, Bristol
Trivedi MH, Lakshmi SM, Joy JM (2012) Pharmacognostic studies of the leaves of Ficus nervosa Heyne ex Roth (Moraceae). Int J Phytother 2:16–22
Kokate CK, Purohit AP, Gokhale SB (1991) Practical Pharmacognosy, 3rd edn. Vallabh Prakashan, New Delhi
Chanda S (2014) Importance of pharmacognostic study of medicinal plants: an overview. J Pharmacog Phytochem 2:69–73
Anonymous (1996) Indian pharmacopoeia, 4th edn. India Ministry of Health and Family Welfare
Kokashi CJ (1958) Fluorescence of powdered vegetable drugs in ultra-violet radiation. J American Pharma Assoc 47:715–717
Chase CR, Pratt RJ (1949) Fluorescence of powdered vegetable drugs. Indian J Exp Biol 33:428–432
Wagner H, Bladt S, Rickl V (2001) Plant drug analysis: a thin-layer chromatography atlas, 2nd edn. Springer, New York, NY
Khandelwal KR (2007) Techniques and experiments, practical Pharmacognosy, 17th edn. Nirali Prakashan, Pune
Ainsworth EA, Gillespie KM (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat Protoc 2:875–877
Alhakmani F, Kumar S, Khan SA (2013) Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 3:623–627
Lin JY, Tang CY (2007) Determination of total phenolic and flavonoid contents in selected fruits and vegetables as well as their stimulatory effect on mouse splenocyte proliferation. Food Chem 101:140–147
Madaan R, Bansal G, Kumar S, Sharma A (2011) Estimation of total phenols and flavonoids in extracts of Actaea spicata roots and antioxidant activity studies. Indian J Pharm Sci 73:666–669
Chandrasekar R, Rao SN (2013) Phytochemical analysis of ethanolic extract of leaves of Leucas indica (EELLI). Int J Pharm Bio Sci 4:33–38
Sampathkumar S, Ramakrishna N (2011) Chromatographic finger print analysis of Naringi crenulata by HPTLC technique. Asian Pac J Trop Biomed 1:S195–S191
Akbar S, Hanif U, Ali J, Ishtiaq S (2014) Pharmacognostic studies of stem, roots and leaves of Malva parviflora L. Asian Pac J Trop Biomed 4:410–415
Harborne JB (1998) Phytochemical methods, 3rd edn. Chapman and Hall, London
Bhole RP, Shinde SS, Chitlange SS, Wankhede SB (2015) A high-performance thin-layer chromatography (HPTLC) method for simultaneous determination of diphenhydramine hydrochloride and naproxen sodium in tablets. Anal Chem Insights 10:47–51
ICH, International conference on harmonization of technical requirements for registration of pharmaceuticals for human use; Validation of analytical procedures: text and methodology, Q2 (R1), Geneva, 2005
Jain P, Jain S, Sharma S, Paliwal S (2019) Pharmacognostical specification and validated high-performance thin-layer chromatographic method for the estimation of quercetin in Phoenix sylvestris root. J Planar Chromatogr 32:31–39
Siddiqui NA, Mothana RA, Al-Rehaily AJ, Alam P, Yousaf M, Ahmed S, Alatar A (2017) High-performance thin-layer chromatography based concurrent estimation of biomarkers ent-phyllanthidine and rutin in the dried aerial parts of Flueggea virosa. Saudi Pharm J 25:696–702
Beghdad MC, Benammar C, Bensalah F, Sabri FZ, Belarbi M, Chemat F (2014) Antioxidant activity, phenolic and flavonoid content in leaves, flowers, stems and seeds of mallow (Malva sylvestris L.) from North Western of Algeria. African J Biotech 13:486–491
Alam F, Saqib QNU (2015) Pharmacognostic study and development of quality control parameters for fruit bark and leaf of Zanthoxylum armatum (Rutaceae). Anc Sci Life 34:147–155
World Health Organization, Quality assurance pharmaceuticals: A compendium of guidelines and related materials, good manufacturing practices and inspection, 2, WHO, Geneva, 1996
Gangwar AK, Ghosh AK, Saxena V (2014) Antiulcer activity of Phoenix dactylifera Linn leaves. World J Pharm Sci 3:1164–1172
Iqbal M, Khera RA, Hussain T, Sadia H, Abbas M, Nazir A, Arshad M, Younas U (2017) Cytotoxicity and bioactivity evaluation of pygmy date palm extracts. Curr Sci Perspect 3:106–111
Acknowledgments
Authors are highly thankful to Vice Chancellor, Banasthali Vidyapith, Banasthali, along with the Department of Science and Technology (DST) and Ministry of Human Resource Development (MHRD), New Delhi, India, for providing the necessary facilities to carry out the entire research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jain, P.K., Jain, S., Chak, P. et al. High-performance thin-layer chromatographic investigation of rutin in the leaves of Phoenix sylvestris in sequence with pharmacognostical and phytochemical evaluation. JPC-J Planar Chromat 33, 191–201 (2020). https://doi.org/10.1007/s00764-020-00016-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00764-020-00016-1