Abstract
Perinatal depression (PND) screening recommendations are made by national, state-based and professional organisations; however, there is disagreement regarding screening timing, provider responsible, screening setting, screening tool as well as the follow-up and referral pathways required post-screening. This systematic review aimed to identify, describe and compare PND screening recommendations from member countries of the Organisation for Economic Co-operation and Development (OECD). Publications were identified through systematically searching PubMed, Google and the Guidelines International Network (GIN). Recommendations regarding PND screening endorsement, timing, frequency, responsible provider, tools/assessments and follow-up and referral were extracted. Twenty-one publications, including guidelines, from five countries were included. Most made recommendations in support of PND screening using the Edinburgh Postnatal Depression Scale. Details differed regarding terminology used, as well as frequency of screening, follow-up mechanisms and referral pathways. A broad range of health providers were considered to be responsible for screening. This is the first review to identify and compare PND screening recommendations from OECD member countries; however, only online publications published in English, from five countries were included. Heterogeneity of publication types and inconsistency in definitions rendered quality assessment inappropriate. While most publications generally endorsed PND screening, there are exceptions and the associated details pertaining to the actual conduct of screening vary between and within countries. Developing clear, standardised recommendations based on current evidence is necessary to ensure clarity amongst healthcare providers and a comprehensive approach for the early detection of PND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perinatal depression (PND) — an episode of depression occurring during pregnancy or the first postpartum year (Bauer et al. 2014) — affects approximately 10–20% of women globally (AIHW 2012; Selix & Goyal 2018; World Health Organization 2021). There is a lack of consistency across official diagnostic criteria when defining PND, especially regarding the postnatal period (Howard & Khalifeh 2020). The DSM-5 uses a “peripartum” specifier when the onset of a depressive disorder occurs during pregnancy, or in the 4 weeks following delivery (American Psychiatric Association 2013), while the International Classification of Diseases 11th Revision (ICD-11) defines the onset of a mental disorder in the puerperium as that which “commences within about 6 weeks after delivery” (World Health Organisation 2020). These “official” diagnoses often differ significantly to clinical opinion and longitudinal studies demonstrating that women are at risk of developing mental health problems up to 5 months postpartum (Munk-Olsen et al., 2006), and published literature often alludes to the postnatal period as up to 12 months postpartum (El-Den et al. 2015; Selix & Goyal 2018). The lack of consensus as to what constitutes the postpartum period may contribute to variability in PND screening recommendations and practices.
PND often goes unidentified with many women not recognizing that their symptoms could be indicative of a mental illness, resulting in a lack of diagnosis and treatment (Cox et al. 2016). Screening has been proposed to allow for earlier identification and appropriate referral for diagnosis and treatment.
PND is associated with poorer health outcomes for both the mother and the baby (Stein et al. 2014), such as an increased risk of low birthweight and preterm birth antenatally (Dadi et al., 2020) and adverse infant developmental outcomes postnatally (O'Hara 2009). There are effective, evidence-based treatment options for PND including psychotherapy and antidepressant medication (O'Connor et al. 2016). Therefore, early identification may allow for timely management and treatment, thereby potentially reducing the risk of these adverse outcomes (Pearlstein et al. 2009). Screening for postnatal depression has been evaluated, with evidence to demonstrate reduced depression risk among women who participate in screening programs (O'Connor et al. 2016). Furthermore, PND screening can lead to increased rates of referrals and service use (Reilly et al. 2020).
Nonetheless, there is debate in the literature as to whether PND screening should be provided routinely (AE Buist et al. 2002; Hazell Raine et al. 2020), due to a lack of evidence demonstrating the effectiveness of screening in improving patient outcomes (Thombs et al. 2014). A systematic review published in 2014 reported that the evidence did not demonstrate effectiveness of PND screening and that robust randomized controlled trials (RCTs) exploring effectiveness and cost-effectiveness are necessary (Thombs et al. 2014). Furthermore, it is important to note that screening for PND, specifically using screening tools such as the Edinburgh Postnatal Depression Scale (EPDS) does not allow for a diagnosis of a depressive disorder, nor does it allow for the identification of women experiencing other mental illnesses, which may be less common, such as personality disorders, bipolar disorder and postpartum psychosis (Boyce & Judd 2019; Judd et al. 2017). Rather, screening, using tools such as the EPDS, can identify those who require further assessment for diagnosis of depression and/or anxiety. Moreover, the psychometric properties of the screening tool also need to be considered in relation to the illness for which it is being used (AE Buist et al. 2002). For example, the EPDS has been used to screen for both anxiety and depressive disorders (Matthey 2008). A screening tool with poor reliability and validity can lead to false positives and ultimately overdiagnosis, as well as false negatives which can be costly (Thombs et al. 2014). Reviews exploring the psychometric properties of depression screening tools in primary care settings, generally (S. El-Den et al. 2018a) and specifically in relation to PND, demonstrate that the EPDS and the Patient Health Questionniare-9 (PHQ-9) are valid and reliable screening tools across various settings when used by a range of trained healthcare professionals (Levis et al. 2020; Wang et al. 2021).
Concerns surrounding screening also often include debate as to whether routine screening is acceptable (AE Buist et al. 2002); however, a systematic review exploring acceptability among key stakeholders, demonstrated that screening is generally acceptable to perinatal women and health professionals (El-Den et al. 2015). There is a growing body of evidence relating to PND screening by a range of health care professionals, such as physicians (Ford et al. 2017), nurses (Segre et al. 2010), midwives (Martin et al. 2020), paediatricians (Byatt et al. 2013; Chambers et al. 2019; Currie & Rademacher 2004) and pharmacists (El-Den et al. 2019; Sarira El-Den et al. 2018a, b, c; Elkhodr et al. 2018); hence, it is often unclear which provider is responsible for screening and there is growing support for integrating early detection in all “medical settings that encounter perinatal women” (Flynn et al. 2006). If screening is to be delivered in a variety of medical settings, then in addition to ensuring healthcare professionals working in those settings are trained, there is a need to also ensure that appropriate, site-specific follow-up and referral pathways for diagnostic assessment and treatment are also established.
Considering the lack of consensus relating to PND screening, the aim of the current review was to identify and compare recommendations relating to PND screening across countries that are members of the Organisation for Economic Co-operation and Development (OECD).
Methods
This systematic review was guided by the PRISMA statement (Moher et al. 2009; Page et al. 2021).
Search strategy
Recommendations pertaining to PND screening from the 37 countries that were members of the OECD in September 2020 (OECD 2020) were identified by searching PubMed, the World Wide Web via Google search engine and the Guidelines International Network (GIN) library. The detailed search strategy for each search is presented in Table 1. The searches were conducted in September 2020, and no date restrictions were applied.
The Google search was repeated for each of the 37 OECD member countries, whereby for each search, the URLs, country of origin, publishing organisation/journal and document/page title for the first 40 results were extracted and exported to an Excel spreadsheet.
While screening and extracting data from full-text citations (e.g. online documents, webpages), if in-text reference to a potentially relevant publication was identified, co-authors attempted to find it and screen it as well.
Inclusion and exclusion criteria
Publications from all OECD member countries, regardless of the issuing organisation (e.g. body, college, committee, institution, association, society, department), were eligible for inclusion, provided that they were published online (at the time of searching and screening), and it was clear (e.g. on the title page) that the recommendation was published by or on behalf of an organisation within an OECD member country, or specifically stipulated that it was intended for adoption within an OECD member country. Provided these criteria were met, a publication was considered eligible for inclusion if it made a recommendation relating to screening for depression and/or risk factors for depression during the perinatal period. Terms such as “screening”, “risk assessment”, “early detection”, “enquiry” and “identification” are often used interchangeably in the literature to refer to preventative health care which “aims to reduce the burden of chronic conditions by early identification of people with risk factors or symptoms and applying appropriate interventions” and psychosocial assessment can encompass depression screening (Austin 2014). Hence, if a publication did not mention the term “screening”, specifically, but it could be ascertained that the publication was making a recommendation pertaining to assessing for, asking about or enquiring about depressive symptoms or risk of depression during the perinatal period for the purpose of identification and “applying appropriate interventions” (e.g. referral/follow-up for diagnostic assessment), then it was eligible for inclusion (Austin 2014). A broad range of publication types were eligible for inclusion, such as guidelines, guidance, recommendations, reports, statements (e.g. position statement, policy statement), consultations, opinions (e.g. committee opinion, organisation opinion) and health professional resources (e.g. toolkit, manual, guide, handbook, booklet).
Publications, such as journal articles, whereby the key focus appeared to be to report on primary research studies only, evaluate evidence or conduct a literature review, as well as publications that were abstracts only, were ineligible for inclusion. Publications were also excluded if written in a language other than English. There was no limit on publication date; however, if there were different versions of the same publication available, only the current/most recent publication was included. Publications that only endorsed pre-existing PND screening recommendations (e.g. by other organisations) and did not develop or make their own recommendation were also excluded.
Study selection and data extraction
Publications identified were exported into Excel, where duplicates were removed. Two authors (IA and SY) then screened the titles, abstracts and full-texts for inclusion and discussed any disagreements to reach consensus. Where disagreements could not be resolved, the publications were reviewed by two additional authors (SED and CRG) to reach consensus. During full-text screening and data extraction, if in-text references to other PND screening recommendations were identified, then potentially relevant references were also screened for inclusion.
Data extraction was led by LP and focused on the PND or PND risk screening recommendation, provider responsible, screening tool recommended, screening frequency and follow-up and referral pathways recommended (Table 2). Every attempt was made to extract data that pertained to depression and the perinatal period specifically, especially for publications whereby the scope of the publication was broader than depression (e.g. mental illness) or the perinatal period (e.g. adults). This was to ensure extracted data was relevant to the aims of this review.
When extracting data relating to the recommendation made, care was taken to extract information specifically pertaining to “screening”, wherever possible. When the term “screening” was not used but aforementioned terminology including but not limited to “assessment” or “enquiry” was used instead, then these were extracted. If a mix of terms was used, then care was taken to extract information pertaining to “screening”, only. Recommendations, including those relating to frequency, timing, follow-up and referral, were extracted verbatim where possible (Table 2). It should be noted that in some instances the extent of data available necessitated that the research team summarise key details during extraction into Table 2, as relevant to the aims of this review.
When extracting information relating to the provider responsible, care was taken to use the exact terminology used in each publication. When the title of the recommendation was targeted towards a specific healthcare profession (e.g. if the recommendations were developed for GPs specifically), then it was assumed that they would be responsible for screening, unless otherwise stated. In the event where extensive follow-up and referral recommendations are made, these are summarised in Table 2. Where any of this information is not reported, “not specified” is stated in Table 2.
Results
Search results and screening
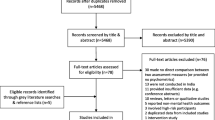
The search yielded 1592 publications, of which 1480 were from Google; 17 were from PubMed; and 95 were from GIN. A further 11 publications were identified during screening and data extraction (citation searching). After duplicate removal, publications were screened based on title, abstract (when available) and then full-text. The screening process yielded 21 included publications (Fig. 1).

Abbreviations: GIN, Guidelines International Network; OECD, Organisation for Economic Co-operation and Development; PND, perinatal depression
Flow chart of article selection process, adapted from the PRISMA 2020 flow diagram for new systematic reviews (Page et al. 2021)
General characteristics
Publications originated from the United States (US) (n = 7) (ACOG 2018; American College of Nurse-Midwives 2020; Earls et al. 2019; Mental Health America 2018; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; Siu et al. 2016; Trangle et al. 2016), Australia (n = 6) (Australian Government Department of Health 2019; COPE 2017; NSW Department of Health 2009; RACGP 2016; RANZCOG 2015; WA Department of Health 2015), Canada (n = 5) (Alberta Health Services 2019; BC Ministry of Health 2008; BC Reproductive Mental Health Program 2014; CTFPHC 2013; Registered Nurses’ Association of Ontario 2018), the United Kingdom (UK) (n = 2) (NICE 2018; SIGN 2012) and New Zealand (n = 1) (New Zealand Guidelines Group 2008). Eleven (ACOG 2018; Alberta Health Services 2019; American College of Nurse-Midwives 2020; BC Reproductive Mental Health Program 2014; COPE 2017; Earls et al. 2019; Mental Health America 2018; NICE 2018; RANZCOG 2015; Registered Nurses’ Association of Ontario 2018; SIGN 2012) publications addressed perinatal mental health, of which seven were specific to perinatal depression, anxiety or mood disorders (ACOG 2018; Alberta Health Services 2019; BC Reproductive Mental Health Program 2014; Earls et al. 2019; RANZCOG 2015; Registered Nurses’ Association of Ontario 2018; SIGN 2012). Five (BC Ministry of Health 2008; CTFPHC 2013; New Zealand Guidelines Group 2008; Siu et al. 2016; Trangle et al. 2016) publications addressed depression in adults, of which two (CTFPHC 2013; Siu et al. 2016) were specific to depression screening. Two publications (NSW Department of Health 2009; WA Department of Health 2015) addressed family mental health; another two publications (Australian Government Department of Health 2019; Obstetric Care Consensus No. 8: Interpregnancy Care 2019) focused on pregnancy care; and one publication (RACGP 2016) focused on preventative health care in adults. Fourteen publications (ACOG 2018; American College of Nurse-Midwives 2020; Australian Government Department of Health 2019; COPE 2017; CTFPHC 2013; Earls et al. 2019; Mental Health America 2018; New Zealand Guidelines Group 2008; NICE 2018; O'Connor et al. 2016; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; RACGP 2016; RANZCOG 2015; SIGN 2012) were published by national organisations; and seven (Alberta Health Services 2019; BC Ministry of Health 2008; BC Reproductive Mental Health Program 2014; NSW Department of Health 2009; Registered Nurses’ Association of Ontario 2018; Trangle et al. 2016; WA Department of Health 2015) publications were published by state/province-based organisations (Table 2).
Recommendations
As can be seen in Table 2, there are variations in the terminology used when making PND screening recommendations, which may have implications for how the recommendations are used and implemented. Sixteen publications recommended screening for depression, or perinatal mood disorders or symptoms of these illnesses specifically and often specified a recommended screening tool (ACOG 2018; Alberta Health Services 2019; American College of Nurse-Midwives 2020; Australian Government Department of Health 2019; BC Ministry of Health 2008; BC Reproductive Mental Health Program 2014; COPE 2017; Earls et al. 2019; Mental Health America 2018; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; RACGP 2016; RANZCOG 2015; Registered Nurses’ Association of Ontario 2018; Siu et al. 2016; Trangle et al. 2016; WA Department of Health 2015). Some publications were less directive, in that “screening” was not recommended specifically, rather recommendations that healthcare professionals “enquire about depressive symptoms” (SIGN 2012), “consider asking depression identification questions” (NICE 2018) or “consider the use of the verbal 2–3 question screening tool” (New Zealand Guidelines Group 2008) were made. However, the use of screening tools such as the EPDS was still often suggested. One publication recommended conducting “psychosocial assessment” (NSW Department of Health 2009), recognising that psychosocial assessment includes depression screening or recommending the use of depression screening tools. The Canadian Task Force on Preventive Health Care (CTFPHC) recommended against routine screening, citing a lack of evidence and noting that “undesirable effects probably outweigh the desirable effects” (CTFPHC 2013). This recommendation was graded as “weak” with “very low quality evidence” (CTFPHC 2013). Nonetheless, being “alert to the possibility of depression” was still recommended in this publication (CTFPHC 2013). Among publications that supported screening, some recommended universal screening (BC Ministry of Health 2008; NSW Department of Health 2009; WA Department of Health 2015) and/or screening for “all” women (ACOG 2018; Alberta Health Services 2019; American College of Nurse-Midwives 2020; BC Reproductive Mental Health Program 2014; Registered Nurses’ Association of Ontario 2018), while others recommended opportunistic screening (RACGP 2016). Furthermore, there was a variation as to whether publications recommended screening routinely (Alberta Health Services 2019; Earls et al. 2019; New Zealand Guidelines Group 2008; RANZCOG 2015; Registered Nurses’ Association of Ontario 2018; Trangle et al. 2016) or specified a minimum number of times screening should be offered, for example, at least once (ACOG 2018; Australian Government Department of Health 2019; BC Reproductive Mental Health Program 2014; COPE 2017) or twice (WA Department of Health 2015).
Screening tools
Eighteen (ACOG 2018; Alberta Health Services 2019; Australian Government Department of Health 2019; BC Ministry of Health 2008; BC Reproductive Mental Health Program 2014; COPE 2017; Earls et al. 2019; Mental Health America 2018; NICE 2018; NSW Department of Health 2009, 2010; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; RACGP 2016; RANZCOG 2015; SIGN 2012; Siu et al. 2016; Trangle et al. 2016; WA Department of Health 2015) publications included the EPDS among the recommended screening tools, including for screening among populations for whom English is not their first language, as it has been translated into other languages (NSW Department of Health 2009). Six (Alberta Health Services 2019; Australian Government Department of Health 2019; BC Reproductive Mental Health Program 2014; COPE 2017; NSW Department of Health 2009; Registered Nurses’ Association of Ontario 2018) publications recommended a translated version of the EPDS when screening perinatal women of culturally and linguistically diverse backgrounds, of which five (Australian Government Department of Health 2019; BC Reproductive Mental Health Program 2014; COPE 2017; NSW Department of Health 2009; Registered Nurses’ Association of Ontario 2018) noted the need for culturally relevant cut-off scores. However, not all translated versions of the EPDS have been validated (Department of Health Government of Western Australia 2006), with Australian publications highlighting the lack of validated screening tools for people from non-English speaking backgrounds (COPE 2017) and recognizing that the translated EPDS may not account for cultural sensitivity and cannot be considered acceptable nor valid for all populations (WA Department of Health 2015).
The PHQ-9 was among the recommended tools in six (ACOG 2018; Earls et al. 2019; Mental Health America 2018; NICE 2018; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; Trangle et al. 2016) publications, of which five were from the US. One publication recommended using the PHQ-9 for screening women from non-English speaking backgrounds (Trangle et al., 2016). Additionally, the PHQ-2 was recommended in two publications (NICE 2018; Trangle et al. 2016).
The New Zealand and UK National Institute for Health and Care Excellence (NICE) publications (New Zealand Guidelines Group 2008; NICE 2018) recommended asking two depression identification questions, first, followed by a screening tool (e.g. EPDS or PHQ-9) if the “woman responds positively” to either question. The Scottish Intercollegiate Guideline Network (SIGN, 2012) publication recommended the use of EPDS or Whooley questions to “aid clinical monitoring and to facilitate discussion of emotional issues”, rather than for screening explicitly, citing a lack of evidence to support the tools’ “sufficient accuracy” in the perinatal period. The Whooley questions, sometimes not referred to by name but described in-text, were identified amongst included publications (New Zealand Guidelines Group 2008; NICE 2018; SIGN 2012).
Screening timing and frequency
When defining the postpartum period, this was often considered to be up to 1 year postpartum (COPE 2017; NICE 2018). The majority of publications made recommendations across the perinatal period; however, some publications aimed to provide guidance postnatally specifically. For example, publications with a focus on postpartum depression screening, interpregnancy care or perinatal care in paediatric practice were identified (Table 2). Due to their focus on the postnatal period, it was not unexpected that the depression screening recommendations within such publications were often specific to the postnatal period.
The recommendations relating to screening intervals ranged from “as early as practical” (Australian Government Department of Health 2019; COPE 2017; NSW Department of Health 2009), or at “first contact” (New Zealand Guidelines Group 2008; NICE 2018) to 32 weeks (BC Reproductive Mental Health Program 2014) or in the third trimester (WA Department of Health 2015) antenatally and 4 weeks to up to 6 months postnatally (Earls et al. 2019). Some publications did not make specific recommendations for frequency and timing, and there was recognition among included publications that “findings have not been consistent” in regard to this issue (Registered Nurses’ Association of Ontario 2018).
Provider responsible
It is evident from the data presented in Table 2 that a broad range of healthcare providers are considered responsible for screening. The terms used to describe them included “clinicians” (NSW Department of Health 2009; RANZCOG 2015; Siu et al. 2016; Trangle et al. 2016), “practitioners” (New Zealand Guidelines Group 2008; RANZCOG 2015), “all health professionals providing care to women in the perinatal period” (COPE 2017), “healthcare professionals” (NICE 2018; SIGN 2012), “healthcare providers” (American College of Nurse-Midwives 2020; BC Reproductive Mental Health Program 2014) and “provider in any healthcare setting” (Mental Health America 2018). In some publications, it was implied which “clinicians” or “practitioners” were specifically responsible, for example, midwives, child and family health nurses (NSW Department of Health 2009) or obstetricians and gynaecologists (RANZCOG 2015). There was also consideration for the healthcare settings (e.g. mental health, obstetric, paediatric, primary care, emergency, occupational health settings) in which screening could occur (Mental Health America 2018). Recommendations which were more specific about responsible providers were often published by organisation or bodies specific to the profession. For example, the publications by the Royal Australian College of General Practitioners (2016) and the American Academy of Pediatrics (Earls et al. 2019) discussed screening by general practitioner paediatric primary care clinicians, respectively.
Referral and follow-up recommendations
The EPDS cut-off scores for further assessment varied across included publications (Alberta Health Services 2019; Australian Government Department of Health 2019; BC Reproductive Mental Health Program 2014; COPE 2017; RANZCOG 2015). For example, a cut-off score of “13 or more” was recommended (Australian Government Department of Health 2019; COPE 2017), as was a cut-off score of “at least 12” (Alberta Health Services 2019). There was recognition that the EPDS may need to be completed multiple times. For example, one publication suggested that a cut-off score of 12 or more warranted monitoring and that the screen be repeated in 2 weeks with a broader recommendation that a “very high EPDS score should be further investigated” (RANZCOG 2015). Some publications made different recommendations for different cut-off scores, whereby an EPDS score of 12 to 13 required monitoring and possible referral, while an EPDS score of 14 or higher required “diagnostic assessment and treatment” (BC Reproductive Mental Health Program 2014).
A broad range of referral recommendations were made, including to various healthcare professionals (BC Reproductive Mental Health Program 2014; Earls et al. 2019; NICE 2018; NSW Department of Health 2009; Obstetric Care Consensus No. 8: Interpregnancy Care 2019; RANZCOG 2015; SIGN 2012), resources (ACOG 2018; BC Reproductive Mental Health Program 2014; Earls et al. 2019), services (Earls et al. 2019; NSW Department of Health 2009; RANZCOG 2015; SIGN 2012), the development of a care or treatment plan (Alberta Health Services 2019; NSW Department of Health 2009; RANZCOG 2015; Registered Nurses’ Association of Ontario 2018; WA Department of Health 2015), treatment (Mental Health America 2018; RACGP 2016) and/or further assessment (Australian Government Department of Health 2019; COPE 2017; New Zealand Guidelines Group 2008; Siu et al. 2016). Healthcare professionals that were specifically mentioned in referral recommendations included GPs (NICE 2018; NSW Department of Health 2009; RANZCOG 2015; SIGN 2012), psychologists (NSW Department of Health 2009; RANZCOG 2015), social workers (RANZCOG 2015) and obstetricians (Earls et al. 2019). Healthcare professionals recommended were also referred to more broadly as “mental health providers” (Obstetric Care Consensus No. 8: Interpregnancy Care 2019) or “mental health professionals” (Earls et al. 2019; NICE 2018), “primary care clinicians” (Earls et al. 2019), or “primary care providers” and “mental health specialists” (BC Reproductive Mental Health Program 2014).
Services recommended included “community adult mental health services” (NSW Department of Health 2009), “services in their local area”(RANZCOG 2015) and “mental health services” (SIGN 2012). Resources recommended included “behavioural health resources” (ACOG 2018), “emergency resource” (BC Reproductive Mental Health Program 2014) as well as “mental health crisis services” and “emergency medical services” (Earls et al. 2019). Publications also often referred to local protocol and policy when suggesting further assessment (Australian Government Department of Health 2019; COPE 2017) and some acknowledged the need to consider depression severity and comorbidities (Siu et al. 2016).
Discussion
Most publications identified in this systematic review recommended PND screening for all women, routinely. However, the guidance provided varied in relation to the terminology used, timing and frequency of screening, screening tool to be used, the health care provider responsible and the appropriate follow-up and referral pathways. Even though most publications supported the detection of PND, some did not recommend “screening” specifically in their recommendations, acknowledging the limited evidence, especially in relation to the effectiveness of screening, benefit of screening, specifics of timing and frequency of screening and responsibility for screening, as well as a lack of established pathways for post-screening referrals (BC Reproductive Mental Health Program 2014; Registered Nurses’ Association of Ontario 2018; SIGN 2012; Siu et al. 2016).
This systematic review identified a lack of consensus in relation to the recommended timing and frequency of PND screening. Screening at first contact during pregnancy was often recommended (COPE 2017; Department of Health and Human Services 2019; Registered Nurses’ Association of Ontario 2018) as the appropriate timing for antenatal screening. The lack of consistency in timing of screening recommendations postnatally may be a reflection of the lack of consensus regarding the definition of the postnatal period (Wisner et al. 2010). For example, in the context of depressive disorders, the ICD-11 and DSM-5 (American Psychiatric Association 2013; World Health Organisation 2020) define the postnatal period as 4 or 6 weeks postpartum, whereas published literature alludes to 12 months postpartum (Woolhouse et al., 2015). Conversely, the term “perinatal” is defined as the perinatal period as commencing at “22 completed weeks of gestation” and ending at “seven completed days after birth” by the World Health Organisation and has been used to describe foetal and neonatal outcomes, rather than maternal outcomes (World Health Organisation 2006), further adding to the confusion in terminology. This may be further complicated by studies that have investigated the longer-term prevalence of depression, such as at 3 and 5 years postpartum, that have reported similar prevalence rates of depression to those reported in studies up to 12 months postpartum (Najman et al. 2000; Wang et al. 2011; Woolhouse et al. 2015). Guidance on PND screening after the 12-month postpartum period was not identified, raising the question as to when the postpartum period ends and the maternal period begins and indicating a need for standardizing terminology, as well as exploring the utility of screening tools in the longer term. Furthermore, historically, terms including “woman” and “mother” are used when referring to people experiencing PND; however, it is important to recognise that those experiencing PND may not always identify as “woman”, “she” or “mother” (Registered Nurses’ Association of Ontario 2018). Awareness of and sensitivity when using appropriate pronouns and terms, which may include “person” or “parent”, are particularly important. Future research into tailored care and appropriate terminology is needed to ensure inclusivity and reduce barriers to healthcare.
Not surprisingly, most of the included publications recommended the use of the EPDS for PND screening due to its effectiveness, validity or reliability, availability in languages other than English as well as ease of use and brevity. While the EPDS is a simple screening tool, healthcare professionals need to be trained in its use to be able to score and interpret responses (Thombs et al. 2014) as well as make decisions regarding optimal cut-off points based on the aim of screening, so as to maximise sensitivity, specificity or both (Levis et al. 2020). Furthermore, its reliability and validity as well as its appropriateness when used with culturally and linguistically diverse populations need to be considered. For example, cultural concerns have been reported in relation to using the EPDS among First Nations mothers; however, its cultural validity may vary across countries, demonstrating the need for further research regarding its use with diverse populations (Chan et al. 2021). Nonetheless, the EPDS has been used by a broad range of healthcare professionals, including general practitioners, obstetricians, paediatricians, midwives, nurses and multicultural health workers to screen for PND across diverse populations and, it has been found to be acceptable in a variety of contexts (El-Den et al. 2015).
This systematic review identified heterogeneity in recommendations pertaining to which healthcare provider was responsible for screening, specifically. Screening should be provided in universal, accessible care settings, such as primary care (Howard & Khalifeh 2020), which encompasses a range of healthcare settings, including but not limited to family physicians/GP practices and community pharmacy and PND screening by a broad range of healthcare professionals who interact with women in the perinatal period, including but not limited to paediatricians and nurses, is acceptable (El-Den et al. 2015). Hence, if screening and follow-up are to be integrated in “all medical settings that encounter perinatal women” (Flynn et al. 2006), then healthcare professionals working in these settings require sufficient training to explain the rationale for screening, conduct screening, interpret and explain screening scores and triage for timely diagnosis and management (A Buist et al. 2007; El-Den et al. 2015). While the role of some specific healthcare professionals, such as pharmacists, was not specifically mentioned in regard to screening, with terminology alluding to “all” and “primary” healthcare professionals within included publications, perhaps their role in screening is implied in some recommendations. However, healthcare professionals frequently report that formal perinatal mental health education is insufficient in curricula (Elkhodr et al. 2018; Legere et al. 2017; Sambrook Smith et al. 2019). Hence, there is a need to develop, evaluate and integrate purpose-designed education with input from mental health professionals and consumers. Preliminary research among trained pharmacy students indicates that they were capable of assessing for suicide risk (82.4%) and referring to appropriate healthcare professionals (88.2%), as demonstrated during role-plays with simulated patients exhibiting symptoms of postnatal depression (S. El-Den et al. 2018b), suggesting that integrating perinatal mental health education in primary healthcare curricula may allow future healthcare professionals to contribute to early detection and referral.
The CTFPHC (2013) explicitly recommended against routine screening for PND; however, the publication did note that “clinicians should be alert to the possibility of depression”, especially for those who might be at high risk. This recommendation against screening was based on a review published in 2012 which included 5 studies, all of which were conducted by the same author(s) in rural regions of Japan among elderly populations, as no other studies were considered eligible for inclusion (Keshavarz et al., 2012). While, a more recent review of 14 studies, with broader inclusion criteria, reported that PND screening was beneficial “in terms of increasing referral to, and engagement with, appropriate support services”, it also acknowledged the need for further research exploring long-term outcomes (Reilly et al. 2020). Furthermore, previous reviews have demonstrated that there is a lack of evidence to demonstrate that screening for PND benefits perinatal women, highlighting the need for high-quality RCTs in this area (Thombs et al. 2014). Moreover, a systematic review exploring the cost-effectiveness of interventions for PND and anxiety could not draw conclusions due to the heterogeneity across studies, but reported that interventions incorporating identification and treatment were likely to be cost-effective (Camacho and Shields 2018). The cost of perinatal mental health problems also needs to be considered in such evaluations, with estimates indicating that the estimated costs of perinatal mental health problems are £8.1 billion per year’s birth in the UK (Bauer et al. 2014) and $877 million per annum for depression and anxiety in Australia, indicating a need to develop a “high quality and comprehensive screening program…in collaboration with health professionals and consumers” (PwC Consulting Australia 2019).
Despite evidence of screening effectiveness in terms of reduced depression risk and increased service use (O'Connor et al. 2016; Reilly et al. 2020), the impact of PND screening on improving detection and management of depression is likely to be low if appropriate referral and follow-up pathways are not integrated for diagnosis and treatment (Hazell Raine et al. 2020; Siu et al. 2016). Among some included publications, screening was often recommended under the assumption that follow-up, care and referral pathways are established and available (BC Reproductive Mental Health Program 2014). For example, the USPSTF recommended screening be “implemented with adequate systems in place to ensure accurate diagnosis, effective treatment and appropriate follow-up” (Siu et al. 2016). However, it is unclear how to determine whether such assumptions have been met and who is responsible to ensure these pathways do exist, making decision-making difficult for the individual healthcare professional. Furthermore, even in high income countries there is often a lack of mental health services and workforce shortages that render referrals even more difficult. For example, women in approximately half of the UK do not have access to specialist perinatal mental health services (Bauer et al. 2014), and there is a “critical shortage” of perinatal mental health service and assessment pathways in Australia (Hazell Raine et al. 2020). Decisions to recommend against screening or to refuse/withdraw funding for screening may be due to the lack of established post-screening pathways, but may also be due to a lack of evidence to demonstrate the sustainability of screening outcomes and potential long-term benefits of screening among children (Hazell Raine et al. 2020). Hence, there is a need for high-quality, well-designed studies exploring the effectiveness of PND screening, by a range of healthcare professionals across healthcare settings, to determine pathways for referral, diagnosis, treatment and recovery as well as the long-term impact and cost-effectiveness of PND screening.
Finally, it is important to also recognize that, in some instances, differences in recommendations pertaining to PND screening may be necessary. There are sometimes vast differences across countries, in terms of availability of trained healthcare workforce, settings and pathways for screening as well as prevalence of and risk factors for depression; and these differences can also exist within a country. Hence, there is often a need for PND screening and treatment recommendations that are specific to certain locations and populations (Hansotte et al., 2017). Nonetheless, it is evident from the current review that further clarity is needed for individual healthcare professionals to be able to use these recommendations to guide their clinical practice and for the development of a comprehensive approach to the early detection of PND based on current evidence. In line with findings of a review, exploring postnatal depression screening and treatment for low-income women within Western countries, the current review also highlights the importance of considering how recommendations will actually be implemented, especially among vulnerable populations (Hansotte et al., 2017). Further research focusing on implementation of screening recommendations across diverse settings and populations can facilitate the development and updating of guidelines, policies and recommendations in this area.
Strengths and limitations
Findings should be interpreted while considering the potential limitations of this systematic review. Due to the heterogeneity in publication types and lack of consistency in terminology used across publications, it was not possible to conduct a quality assessment. Another potential limitation is that that this systematic review only identified guidelines and recommendations from five out of 37 countries that were members of the OECD at the time of searching, which may be due to only including publications published in the English language which were available online at the time searching, screening and extraction were conducted. It is possible that other guidelines and recommendations have become available online or have been published or updated, since the current review was conducted and that these were not captured. It is also possible that webpages were updated during the months of screening and extraction, and this may have influenced the publications that were ultimately included in this systematic review; however, this is the nature of the online environment from which included publications were identified. Furthermore, while every effort was made to include any publication issued by a body within an OECD member country that included a PND screening recommendation, adherence to the inclusion and exclusion criteria by research team members may have resulted in the exclusion of publications at the title or abstract stage which did not make clear their intention to make recommendations within the body of the text. Future research exploring recommendations published in other languages, through other mediums and by other authors, as well as those issued by countries that are not members of the OECD is warranted.
As stated in “Methods”, where possible data extraction is verbatim; however, when needed, the authors of this review had to summarise data from included publications to ensure data extraction was relevant to the review aims, as well as, for the purpose of clarity, consistency and conciseness in Table 2. While care and effort were taken to ensure no assumptions were made on behalf of an individual publication’s authors, it is possible that differences in terminology and understanding, as well as, publication structure and flow resulted in errors in data extraction. Nonetheless, to the authors’ knowledge, the strength of this review is that it is the first to aim to systematically identify and compare PND screening recommendations, paving the way for further research and inquiry in this area.
Conclusion
This systematic review identified 21 publications from OECD member countries, of which the majority supported PND screening. Recommendations relating to the timing, frequency, screening tool and healthcare provider responsible varied. There is a need to establish and evaluate referral and follow-up pathways. While evidence of the effectiveness of screening is limited, given the high prevalence of PND and the potential negative consequences of undetected and unmanaged depression on parents and children, ensuring PND is detected, referred and managed early is essential. Nonetheless, screening and associated referral services need to be integrated into the broader health system in a manner that allows for coordinated care by multiple healthcare professionals who are involved in the care of perinatal women. Future research exploring the outcomes of screening by a broad range of healthcare professionals and evaluating perinatal mental health education in healthcare curricula is warranted.
References
American College of Obstetitcians and Gynecologists (ACOG) (2018) Screening for perinatal depression. from https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2018/11/screening-for-perinatal-depression
Australian Institute of Heath and Welfare (AIHW) (2012) Perinatal depression: data from the 2010 Australian National Infant Feeding Survey. Retrieved from https://www.aihw.gov.au/reports/primary-health-care/perinatal-depression-data-from-the-2010-australia
Alberta Health Services (2019) Postpartum depression screening. 2020, from https://extranet.ahsnet.ca/teams/policydocuments/1/clp-prov-public-health-well-child-ppd-screen-guideline-hcs-229-01.pdf
American College of Nurse-Midwives (2020) Position statement: mental health during childbirth and across the lifespan: American College of Nurse-Midwives.
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-V Available from https://doi.org/10.1176/appi.books.9780890425596
Austin MP (2014) Marcé International Society position statement on psychosocial assessment and depression screening in perinatal women. Best Pract Res Clin Obstet Gynaecol 28(1):179–187
Australian Government Department of Health. (2019). Clinical practice guidelines pregnancy care. from https://www.health.gov.au/sites/default/files/pregnancy-care-guidelines_0.pdf
Bauer A, Parsonage M, Knapp M et al (2014) The costs of perinatal mental health problems. London
BC Ministry of Health (2008) Family physician guide: for depression, anxiety disorders, early psychosis and substance use disorders. from https://www.health.gov.bc.ca/library/publications/year/2008/fpg_full.pdf
BC Reproductive Mental Health Program, PSB (2014) Best practice guidelines for mental health disorders in the perinatal period
Boyce PM, Judd F (2019) Screening for perinatal depression: is it enough? Med J Aust 210(1):19–20
Buist AE, Barnett BE, Milgrom J et al (2002) To screen or not to screen–that is the question in perinatal depression. Med J Aust 177(S7):S101-105
Buist A, Ellwood D, Brooks J et al (2007) National program for depression associated with childbirth: the Australian experience. Best Pract Res Clin Obstet Gynaecol 21(2):193–206
Byatt N, Biebel K, Friedman L et al (2013) Women’s perspectives on postpartum depression screening in pediatric settings: a preliminary study. Arch Womens Ment Health 16(5):429–432
Camacho EM, Shields GE (2018) Cost-effectiveness of interventions for perinatal anxiety and/or depression: a systematic review. BMJ Open 8(8):e022022
Chambers JE, Denne SC, On behalf of the Pediatric Policy Council (2019) Screening for postpartum depression: obligation and opportunity for pediatricians to improve the lives of children. Pediatr Res 85(7):923-924
Chan AW, Reid C, Skeffington P et al (2021) A systematic review of EPDS cultural suitability with indigenous mothers: a global perspective. Arch Womens Ment Health 24(3):353–365
Centre of Perinatal Excellence (COPE) (2017) Mental health care in the perinatal period: Australian Clinical Practice Guideline. from https://www.cope.org.au/wp-content/uploads/2018/05/COPE-Perinatal-MH-Guideline_Final-2018.pdf
Cox EQ, Sowa NA, Meltzer-Brody SE et al (2016) The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry 77(9):1189–1200
Canadian Task Force on Preventive Health Care (CTFPHC) (2013) Recommendations on screening for depression in adults. Can Med Assoc J 185(9):775–782
Currie ML, Rademacher R (2004) The pediatrician’s role in recognizing and intervening in postpartum depression. Pediatric clinics of North America, 51(3), 785–801, xi
Dadi AF, Miller ER, Bisetegn TA et al (2020) Global burden of antenatal depression and its association with adverse birth outcomes: an umbrella review. BMC Public Health 20(1):173
Department of Health and Human Services. (2019). Perinatal mental health and psychosocial assessment: practice resource manual for Victorian maternal and child health nurses. from https://www2.health.vic.gov.au/Api/downloadmedia/%7B9980990C-A207-49A5-A592-8C53FDA46127%7D
Department of Health Government of Western Australia. (2006). Edinburgh postnatal depression scale (EPDS): translated versions - validated. 2020, from https://www.mcpapformoms.org/Docs/Edinburgh%20Depression%20Scale%20Translated%20Government%20of%20Western%20Australia%20Department%20of%20Health.pdf
Earls MF, Yogman MW, Mattson G et al (2019) Incorporating recognition and management of perinatal depression into pediatric practice. Pediatrics 143(1)
El-Den S, O’Reilly CL, Chen TF (2015) A systematic review on the acceptability of perinatal depression screening. J Affect Disord 188:284–303
El-Den S, Chen TF, Gan YL et al (2018a) The psychometric properties of depression screening tools in primary healthcare settings: a systematic review. J Affect Disord 225:503–522
El-Den S, Chen TF, Moles RJ et al (2018b) Assessing mental health first aid skills using simulated patients. Am J Pharm E 82(2):6222
El-Den S, O’Reilly CL, Gardner DM et al (2019) Content validation of a questionnaire measuring basic perinatal depression knowledge. Women Health 59(6):615–630
El-Den S, O’Reilly CL, Chen TF (2018c) Development and psychometric evaluation of a questionnaire to measure attitudes toward perinatal depression and acceptability of screening: the PND Attitudes and Screening Acceptability Questionnaire (PASAQ). Eval Health Prof 0163278718801434
Elkhodr S, Saba M, O’Reilly C et al (2018) The role of community pharmacists in the identification and ongoing management of women at risk for perinatal depression: a qualitative study. Int J Soc Psychiatry 64(1):37–48
Flynn HA, Blow FC, Marcus SM (2006) Rates and predictors of depression treatment among pregnant women in hospital-affiliated obstetrics practices. Gen Hosp Psychiatry 28(4):289–295
Ford E, Shakespeare J, Elias F et al (2017) Recognition and management of perinatal depression and anxiety by general practitioners: a systematic review. Fam Pract 34(1):11–19
Hansotte E, Payne SI, Babich SM (2017) Positive postpartum depression screening practices and subsequent mental health treatment for low-income women in Western countries: a systematic literature review. Public Health Rev 38:3
Hazell Raine K, Thorpe K, Boyce P (2020) Perinatal depression screening in Australia: a position paper. [https://doi.org/10.1111/nhs.12793]. Nursing & Health Sciences, n/a(n/a)
Howard LM, Khalifeh H (2020) Perinatal mental health: a review of progress and challenges. World Psychiatry 19(3):313–327
Judd F, Newman LK, Komiti AA (2017) Time for a new zeitgeist in perinatal mental health. Aust N Z J Psychiatry 52(2):112–116
Keshavarz H, Fitzpatrick-Lewis D, Streiner D et al (2012) Screening for depression: a summary of the evidence for the Canadian Task Force on Preventive Health Care. Hamilton (ON): McMaster Evidence Review and Synthesis Centre
Legere LE, Wallace K, Bowen A et al (2017) Approaches to health-care provider education and professional development in perinatal depression: a systematic review. BMC Pregnancy Childbirth 17(1):239
Levis B, Negeri Z, Sun Y et al (2020) Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ 371:m4022
Martin PCJH, Norris DG, Martin PCR (2020) Midwives’ role in screening for antenatal depression and postnatal depression. Br J Midwifery 28(9):666–672
Matthey S (2008) Using the Edinburgh Postnatal Depression Scale to screen for anxiety disorders. Depress Anxiety 25(11):926–931
Mental Health America (2018) Position Statement 49: perinatal mental health. 2021, from https://mhanational.org/issues/position-statement-49-perinatal-mental-health
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151(4):264–269, w264
Munk-Olsen T, Laursen TM, Pedersen CB et al (2006) New parents and mental disorders: a population-based register study. JAMA 296(21):2582–2589
Najman JM, Andersen MJ, Bor W et al (2000) Postnatal depression-myth and reality: maternal depression before and after the birth of a child. Soc Psychiatry Psychiatr Epidemiol 35(1):19–27
New Zealand Guidelines Group (2008) Identification of common mental disorders and management of depression in primary care. Evidence-based best practice guideline., from https://www.health.govt.nz/system/files/documents/publications/depression_guideline.pdf
National Institute for Health and Care Excellence (NICE) (2018) Antenatal and postnatal mental health: clinical management and service guideline. from https://www.nice.org.uk/guidance/cg192/evidence/full-guideline-pdf-4840896925
NSW Department of Health (2009) Families NSW supporting families early package – SAFE START guidelines: improving mental health outcomes for parents and infants. 2020, from https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2010_004.pdf
NSW Department of Health (2010) Maternal & Child Health Primary Health Care Policy
Obstetric Care Consensus No. 8: interpregnancy care (2019) Obstet Gynecol 133(1):e51-e72
O'Connor E, Rossom RC, Henninger M et al (2016) U.S. Preventive Services Task Force Evidence Syntheses, formerly systematic evidence reviews screening for depression in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US)
Organisation for Economic Co-operation and Development (OECD) (2020) National accounts of OECD countries, Volume 2020 Issue 2: Detailed Tables. Paris: OECD Publishing
O’Hara MW (2009) Postpartum depression: what we know. J Clin Psychol 65(12):1258–1269
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Pearlstein T, Howard M, Salisbury A et al (2009) Postpartum depression. Am J Obstet Gynecol 200(4):357–364
PwC Consulting Australia (2019) The cost of perinatal depression and anxiety in Australia. Australia: Gidget Foundation
The Royal Australian College of General Practitioners (RACGP) (2016) Guidelines for preventative activities in general practice Available from https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) (2015) Perinatal anxiety and depression. from https://ranzcog.edu.au/RANZCOG_SITE/media/RANZCOG-MEDIA/Women%27s%20Health/Statement%20and%20guidelines/Clinical-Obstetrics/Perinatal-Anxiety-and-Depression-(C-Obs-48)-Review-March-2015.pdf?ext=.pdf
Registered Nurses’ Association of Ontario (2018) Assessment and interventions for perinatal depression second edition. International Affairs and Best Practice Guidelines. 2021, from https://rnao.ca/sites/rnao-ca/files/bpg/Perinatal_Depression_FINAL_web_0.pdf
Reilly N, Kingston D, Loxton D et al (2020) A narrative review of studies addressing the clinical effectiveness of perinatal depression screening programs. Women and Birth 33(1):51–59
Sambrook Smith M, Lawrence V, Sadler E et al (2019) Barriers to accessing mental health services for women with perinatal mental illness: systematic review and meta-synthesis of qualitative studies in the UK. BMJ Open 9(1):e024803
Segre LS, O’Hara MW, Arndt S et al (2010) Screening and counseling for postpartum depression by nurses: the women’s views. MCN Am J Matern Child Nurs 35(5):280–285
Selix NW, Goyal D (2018) Recent policy changes in perinatal depression screening and treatment. The Journal for Nurse Practitioners 14(2):117–123
Scottish Intercollegiate Guidelines Network (SIGN) (2012) Management of perinatal mood disorders: a national clinical guideline. from https://www.sign.ac.uk/media/1065/sign127_update.pdf
Siu AL, Bibbins-Domingo K, Grossman DC et al (2016) Screening for depression in adults: US Preventive Services Task Force Recommendation Statement. JAMA 315(4):380–387
Stein A, Pearson RM, Goodman SH et al (2014) Effects of perinatal mental disorders on the fetus and child. Lancet 384(9956):1800–1819
Thombs BD, Arthurs E, Coronado-Montoya S et al (2014) Depression screening and patient outcomes in pregnancy or postpartum: a systematic review. J Psychosom Res 76(6):433–446
Trangle M, Gursky J, Haight R et al (2016) Healthcare guideline: depression in primary care. from https://www.icsi.org/wp-content/uploads/2019/01/Depr.pdf
WA Department of Health (2015) Perinatal and infant mental health model of care - a framework. from https://kemh.health.wa.gov.au/-/media/Files/Hospitals/WNHS/Our-Services/State-wide-Services/SPIMHP/Perinatal_and_Infant_Mental_Health_Model_of_Care2016.pdf
Wang L, Wu T, Anderson JL et al (2011) Prevalence and risk factors of maternal depression during the first three years of child rearing. J Womens Health (larchmt) 20(5):711–718
Wang L, Kroenke K, Stump TE et al (2021) Screening for perinatal depression with the Patient Health Questionnaire depression scale (PHQ-9): a systematic review and meta-analysis. Gen Hosp Psychiatry 68:74–82
Wisner KL, Moses-Kolko EL, Sit DK (2010) Postpartum depression: a disorder in search of a definition. Arch Womens Ment Health 13(1):37–40
Woolhouse H, Gartland D, Mensah F et al (2015) Maternal depression from early pregnancy to 4 years postpartum in a prospective pregnancy cohort study: implications for primary health care. BJOG 122(3):312–321
World Health Organisation. (2006). Neonatal and perinatal mortality: WHO.
World Health Organisation (2020) 6E20 Mental or behavioural disorders associated with pregnancy, childbirth or the puerperium, without psychotic symptoms. ICD-11 for Mortality and Morbidity Statistics 2020, from https://icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1124422593
World Health Organization (2021) Maternal health. Mental Health and Substance Use, 2021, from https://www.who.int/teams/mental-health-and-substance-use/maternal-mental-health
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Den, S., Pham, L., Anderson, I. et al. Perinatal depression screening: a systematic review of recommendations from member countries of the Organisation for Economic Co-operation and Development (OECD). Arch Womens Ment Health 25, 871–893 (2022). https://doi.org/10.1007/s00737-022-01249-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-022-01249-1