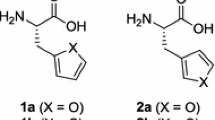
Abstract
The present study describes an efficient access to enantioenriched pyrimidines’ derivatives from readily available Boc-AA-NH2 and β-enaminones. This strategy allows the synthesis of a large variety of chiral pyrimidines (18 examples) with good yields from the chiral pool. In the case of peptide isosteres, this procedure proved to be highly stereoretentive and paves the way to the construction of C-terminal modified peptidomimetics as illustrated in the synthesis of two original pyrimidines containing pseudo-dipeptides.







Similar content being viewed by others
Abbreviations
- AA:
-
Amino acid
- Ac:
-
Acetyl
- Ala:
-
Alanine
- Bn:
-
Benzyl
- Boc:
-
Tert-butoxycarbonyl
- Cbz:
-
Carboxybenzyl
- DCM:
-
Dichloromethane
- Glu:
-
Glutamine
- Gly:
-
Glycine
- Ile:
-
Isoleucine
- KHMDS:
-
Potassium bis(trimethylsilyl)amide
- Met:
-
Methionine
- MS:
-
Molecular sieve
- Phe:
-
Phenylalanine
- Pro:
-
Proline
- PyBOP:
-
Benzotriazol-1-yloxytripyrrolidinophosphonium hexafluorophosphate
- THF:
-
Tetrahydrofuran
- Tyr:
-
Tyrosine
- Val:
-
Valine
References
Arbour CA, Mendoza LG, Stockdill JL (2020) Recent advances in the synthesis of C-terminally modified peptides. Org Biomol Chem 18:7253–7272. https://doi.org/10.1039/D0OB01417F
Bird MJ, Silvestri AP, Dawson PE (2018) Expedient on-resin synthesis of peptidic benzimidazoles. Bioorg Med Chem Lett 28:2679–2681. https://doi.org/10.1016/j.bmcl.2018.04.062
Boeijen A, Liskamp RMJ (1999) Solid-phase synthesis of oligourea peptidomimetics. Eur J Org Chem https://doi.org/10.1002/(SICI)1099-0690(199909)1999:9<2127::AID-EJOC2127>3.0.CO;2-T
Boger DL, Honda T, Dang Q (1994) Total synthesis of bleomycin A2 and related agents. 2. Synthesis of (-)-Pyrimidoblamic acid, epi-(+)-Pyrimidoblamic acid, (+)-Desacetamidopyrimidoblamic acid, and (-)-Descarboxamidopyrimidoblamic acid. J Am Chem Soc 116:5619–5630. https://doi.org/10.1021/ja00092a012
Cheng W-M, Shang R, Fu Y (2017) Photoredox/Brønsted acid co-catalysis enabling decarboxylative coupling of amino acid and peptide redox-active esters with N-heteroarenes. ACS Catal 7:907–911. https://doi.org/10.1021/acscatal.6b03215
Crone WJK, Vior NM, Santos-Aberturas J, Schmitz LG, Leeper FJ, Truman AW (2016) Dissecting bottromycin biosynthesis using comparative untargeted metabolomics. Angew Chem Int Ed 55:9639–9643. https://doi.org/10.1002/anie.201604304
Duerfeldt AS, Boger DL (2014) Total syntheses of (−)-Pyrimidoblamic acid and P-3A. J Am Chem Soc 136:2119–2125. https://doi.org/10.1021/ja412298c
Elboray EE, Grigg R, Fishwick CWG, Kilner C, Sarker MAB, Aly MF, Abbas-Temirek HH (2011) X=Y–ZH compounds as potential 1,3-dipoles. Part 65: atom economic cascade synthesis of highly functionalized pyrimidinylpyrrolidines. Tetrahedron 67:5700–5710. https://doi.org/10.1016/j.tet.2011.05.074
El-Dahshan A, Nazir S, Ahsanullah S, Ansari FL, Rademann J (2011) Peptide–Heterocycle Chimera: New Classes of More Drug-Like Peptidomimetics by Ligations of Peptide–Bis(electrophiles) with Various Bis(nucleophiles). Eur J Org Chem. https://doi.org/10.1002/ejoc.201001206
Ezawa T, Kawashima Y, Noguchi T, Jung S, Imai N (2017) Amidation of carboxylic acids via the mixed carbonic carboxylic anhydrides and its application to synthesis of antidepressant (1S,2R)-tranylcypromine. Tetrahedron Asymmetry 28:1690–1699. https://doi.org/10.1016/j.tetasy.2017.10.015
Gayon E, Szymczyk M, Gérard H, Vrancken E, Campagne JM (2012) Stereoselective and catalytic access to β-enaminones: an entry to pyrimidines. J Org Chem 77:9205–9220. https://doi.org/10.1021/jo301675g
Gayon E, Gérard H, Vrancken E, Campagne JM (2015) Diversity in synthesis of N-heterocycles from simple propargylic alcohols. Synlett 26:2336–2350. https://doi.org/10.1055/s-0034-1378804
Grach G, Pieters G, Dinut A, Terrasson V, Medimagh R, Bridoux A, Razafimahaleo V, Gaucher A, Marque S, Marrot J, Prim D, Gil R, Planas JG, Viñas C, Thomas I, Roblin JP, Troin Y (2011) N-Heterocyclic pyridylmethylamines: synthesis, complexation, molecular structure, and application to asymmetric suzuki-miyaura and oxidative coupling reactions. Organometallics 30:4074–4086. https://doi.org/10.1021/om200375s
Guo W, Zhao M, Tan W, Zheng L, Tao K, Fan X (2019) Developments towards synthesis of N-heterocycles from amidines via C-N/C–C bond formation. Org Chem Front 6:2120–2141. https://doi.org/10.1039/C9QO00283A
Gupta S, Melanson JA, Vaillancourt L, Nugent WA, Tanoury GJ, Schatte G, Snieckus V (2018) Pyrimidine as an Aryl C-H Activating Group Org. Lett 20:3745–3748. https://doi.org/10.1021/acs.orglett.8b01300
Herbert Pucheta JE, Candy M, Colin O, Requet A, Bourdreux F, Galmiche-Loire E, Gaucher A, Thomassigny C, Prim D, Mahfoudh M, Leclerc E, Campagne JM, Farjon J (2015) Understand, elucidate and rationalize the coordination mode of pyrimidylmethylamines: an intertwined study combining NMR and DFT methods. Phys Chem Chem Phys 17:8740–8749. https://doi.org/10.1039/C5CP00241A
Katritzky AR, El-Nache C, Bajaj K, Kubik J, Haase D (2010) Efficient Syntheses of Thiadiazole Peptides. J Org Chem 75:6009–6011. https://doi.org/10.1021/jo100922c
Kersavond TV, Konopatzki R, Chakrabarty S, Blank-Landeshammer B, Sickmann A, Verhelst SHL (2019) Short Peptides with Uncleavable Peptide Bond Mimetics as Photoactivatable Caspase-3 Inhibitors. Molecules 24:206–221. https://doi.org/10.3390/molecules24010206
Lovering F, Bikker J, Humblet C (2009) Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J Med Chem 52:6752–6756. https://doi.org/10.1021/jm901241e
Mahfoudh M, Abderrahim R, Leclerc E, Campagne JM (2017) Recent approaches to the synthesis of pyrimidine derivatives. Eur J Org Chem. https://doi.org/10.1002/ejoc.201700008
Pels K, Kodadek T (2015) Solid-phase synthesis of diverse peptide tertiary amides by reductive amination. ACS Comb Sci 17:152–155. https://doi.org/10.1021/acscombsci.5b00007
Terrasson V, Prim D, Marrot J (2008) N-heterocyclic benzhydrylamines as new N, N-bidentate ligands in palladium complexes: synthesis, characterization and catalytic activity. Eur J Inorg Chem 2008:2739–2745. https://doi.org/10.1002/ejic.200800154
Travin DY, Metelev M, Serebryakova M, Komarova ES, Osterman IA, Ghilarov D, Severinov K (2018) Biosynthesis of translation inhibitor klebsazolicin proceeds through heterocyclization and N-terminal amidine formation catalyzed by a single YcaO enzyme. J Am Chem Soc 140:5625–5633. https://doi.org/10.1021/jacs.8b02277
Trotter NS, Brimble MA, Harris PWR, Callis DJ, Sieg F (2005) Synthesis and neuroprotective activity of analogues of glycyl-l-prolyl-l-glutamic acid (GPE) modified at the α-carboxylic acid. Bioorg Med Chem 13:501–517. https://doi.org/10.1016/j.bmc.2004.10.005
Toumi M, Couty F, Evano G (2008) Total Synthesis of the Cyclopeptide Alkaloid Paliurine E. Insights into Macrocyclization by Ene−Enamide RCM. J Org Chem 73:1270–1281. https://doi.org/10.1021/jo702092x
Valverde IE, Bauman A, Kluba CA, Vomstein S, Walter MA, Mindt TL (2013) 1,2,3-Triazoles as Amide Bond Mimics: Triazole Scan Yields Protease-Resistant Peptidomimetics for Tumor Targeting. Angew Chem Int Ed 52:8957–8960. https://doi.org/10.1002/anie.201303108
Walsh CT, Malcolmson SJ, Young TS (2012) Three ring posttranslational circuses: insertion of oxazoles, thiazoles, and pyridines into protein-derived frameworks. ACS Chem Biol 7:429–442. https://doi.org/10.1021/cb200518n
Wang J, Feng Y, Ji X, Deng G, Leng Y, Liu H (2013) Synthesis and biological evaluation of pyrrolidine-2-carbonitrile and 4-fluoropyrrolidine-2-carbonitrile derivatives as dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes. Bioorg Med Chem 21:7418–7429. https://doi.org/10.1016/j.bmc.2013.09.048
Xu Z, DiCesare JC, Baures PW (2010) Parallel synthesis of an oligomeric imidazole-4,5-dicarboxamide library. J Comb Chem 12:248–254. https://doi.org/10.1021/cc1000105
Yamada T, Yagita M, Kobayashi Y, Sennari G, Shimamura H, Matsui H, Horimatsu Y, Hanaki H, Hirose T, Omura S, Sunazuka T (2108) Synthesis and evaluation of antibacterial activity of bottromycins. J Org Chem 83: 7135–7149. https://doi.org/10.1021/acs.joc.8b00045
Zall A, Bensinger D, Schmidt B (2012) Oxidative homologation of aldehydes to α-ketoaldehydes by using Iodoform, o-Iodoxybenzoic acid, and dimethyl sulfoxide. Eur J Org Chem :1439–1447. https://doi.org/10.1002/ejoc.201101835
Funding
The project was supported by funds from the ENSCM, the CNRS, the Tunisian Ministry of Higher Education and Scientific Research, and the Erasmus Mundus Programme KITE (S.S. mobility grant).
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest/competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahtel, S., Maamer, C.B., Besbes, R. et al. Straightforward synthesis of various chiral pyrimidines bearing a stereogenic center adjacent to the C-2 position, including C-terminal peptide isosteres. Amino Acids 54, 1519–1526 (2022). https://doi.org/10.1007/s00726-022-03192-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03192-y