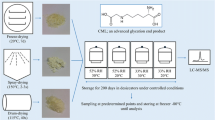
Abstract
Milk processing relies on thermal treatments warranting microbiologically safe products with extended shelf life. However, elevated temperatures favor also Maillard reactions yielding the structurally diverse advanced glycation end products (AGEs). AGEs may alter protein functions and immunogenicity and also decrease the nutritional value of milk products. Furthermore, dietary AGEs contribute to the circulating AGE pool with potentially harmful effects. Here, 14 types of protein-derived AGEs present in raw milk or produced during processing/storage of regular and lactose-free milk products were identified by nanoRP-UPLC-ESI–MS/MS. In total, 132 peptides (118 modification sites in 62 proteins) were modified by at least one studied AGE. Amide-AGEs were the most abundant group with formyllysine being the main type. Most lysine- and arginine-derived AGEs and their modification sites have not been reported before. The number of AGE modification sites increased with the harsher processing conditions of regular milk, but remained stable during storage. This was further supported by quantitative data.



Similar content being viewed by others
References
Aalaei K, Sjöholm I, Rayner M, Tareke E (2017) The impact of different drying techniques and controlled storage on the development of advanced glycation end products in skim milk powders using isotope dilution ESI-LC-MS/MS. Food Bioprocess Tech 10:1704–1714. https://doi.org/10.1007/s11947-017-1936-x
Assar SH, Moloney C, Lima M, Magee R, Ames JM (2009) Determination of N ɛ-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry. Amino Acids 36:317–326. https://doi.org/10.1007/s00726-008-0071-4
Birlouez-Aragon I (2004) Assessment of protein glycation markers in infant formulas. Food Chem 87:253–259. https://doi.org/10.1016/j.foodchem.2003.11.019
Dyer JM, Clerens S, Grosvenor A, Thomas A, Callaghan C, Deb-Choudhury S, Haines S (2016) Proteomic tracking of hydrothermal Maillard and redox modification in lactoferrin and β-lactoglobulin: location of lactosylation, carboxymethylation, and oxidation sites. J Dairy Sci 99:3295–3304. https://doi.org/10.3168/jds.2015-10273
Elmhiri G et al (2015) Formula-derived advanced glycation end products are involved in the development of long-term inflammation and oxidative stress in kidney of IUGR piglets. Mol Nutr Food Res 59:939–947. https://doi.org/10.1002/mnfr.201400722
Förster A, Kühne Y, Henle TO (2005) Studies on absorption and elimination of dietary maillard reaction products. Ann N Y Acad Sci 1043:474–481. https://doi.org/10.1196/annals.1333.054
Geicu OI, Stanca L, Dinischiotu A, Serban AI (2018) Proteomic and immunochemical approaches to understanding the glycation behaviour of the casein and β-lactoglobulin fractions of flavoured drinks under UHT processing conditions. Sci Rep 8:12869. https://doi.org/10.1038/s41598-018-28943-4
Glomb MA, Pfahler C (2001) Amides are novel protein modifications formed by physiological sugars. J Biol Chem 276:41638–41647. https://doi.org/10.1074/jbc.M103557200
Greifenhagen U, Frolov A, Blüher M, Hoffmann R (2016) Plasma proteins modified by advanced glycation end products (AGEs) reveal site-specific susceptibilities to glycemic control in patients with type 2 Diabetes. J Biol Chem 291:9610–9616. https://doi.org/10.1074/jbc.M115.702860
Guy PA, Fenaille F (2006) Contribution of mass spectrometry to assess quality of milk-based products. Mass Spectrom Rev 25:290–326. https://doi.org/10.1002/mas.20074
Hegele J, Buetler T, Delatour T (2008) Comparative LC–MS/MS profiling of free and protein-bound early and advanced glycation-induced lysine modifications in dairy products. Anal Chim Acta 617:85–96. https://doi.org/10.1016/j.aca.2007.12.027
Henle T (2005) Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 29:313–322. https://doi.org/10.1007/s00726-005-0200-2
Henle T, Schwarzenbolz U, Klostermeyer H (1997) Detection and quantification of pentosidine in foods. Zeitschrift für Lebensmitteluntersuchung und -Forschung A 204:95–98. https://doi.org/10.1007/s002170050043
Koschinsky T et al (1997) Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Natl Acad Sci U S A 94:6474–6479
Lima M, Moloney C, Ames JM (2009) Ultra performance liquid chromatography-mass spectrometric determination of the site specificity of modification of β-casein by glucose and methylglyoxal. Amino Acids 36:475–481. https://doi.org/10.1007/s00726-008-0105-y
Meltretter J, Wust J, Pischetsrieder M (2013) Comprehensive analysis of nonenzymatic post-translational beta-lactoglobulin modifications in processed milk by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J Agric Food Chem 61:6971–6981. https://doi.org/10.1021/jf401549j
Meltretter J, Wust J, Pischetsrieder M (2014) Modified peptides as indicators for thermal and nonthermal reactions in processed milk. J Agric Food Chem 62:10903–10915. https://doi.org/10.1021/jf503664y
Milkovska-Stamenova S, Hoffmann R (2016a) Hexose-derived glycation sites in processed bovine milk. J Proteomics 134:102–111. https://doi.org/10.1016/j.jprot.2015.12.022
Milkovska-Stamenova S, Hoffmann R (2016b) Identification and quantification of bovine protein lactosylation sites in different milk products. J Proteomics 134:112–126. https://doi.org/10.1016/j.jprot.2015.07.021
Milkovska-Stamenova S, Hoffmann R (2017) Influence of storage and heating on protein glycation levels of processed lactose-free and regular bovine milk products. Food Chem 221:489–495. https://doi.org/10.1016/j.foodchem.2016.10.092
Milkovska-Stamenova S, Mnatsakanyan R, Hoffmann R (2017) Protein carbonylation sites in bovine raw milk and processed milk products. Food Chem 229:417–424. https://doi.org/10.1016/j.foodchem.2017.02.102
Milkovska-Stamenova S, Krieg L, Hoffmann R (2018) Products of early and advanced glycation in the soy milk proteome. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201800725
Nedic O, Rattan SI, Grune T, Trougakos IP (2013) Molecular effects of advanced glycation end products on cell signalling pathways, ageing and pathophysiology. Free Radic Res 47(Suppl 1):28–38. https://doi.org/10.3109/10715762.2013.806798
Pischetsrieder M, Henle T (2012) Glycation products in infant formulas: chemical, analytical and physiological aspects. Amino Acids 42:1111–1118. https://doi.org/10.1007/s00726-010-0775-0
Poulsen MW et al (2013) Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol 60:10–37. https://doi.org/10.1016/j.fct.2013.06.052
Rabilloud T, Strub J-M, Luche S, Girardet JL, van Dorsselaer A, Lunardi J (2000) Ruthenium II tris (bathophenanthroline disulfonate), a powerful fluorescent stain for detection of proteins in gel with minimal interference in subsequent mass spectrometry analysis. Proteome 1:1–14. https://doi.org/10.1007/s102160000002
Renzone G, Arena S, Scaloni A (2015) Proteomic characterization of intermediate and advanced glycation end-products in commercial milk samples. J Proteomics 117:12–23. https://doi.org/10.1016/j.jprot.2014.12.021
Teodorowicz M, van Neerven J, Savelkoul H (2017) Food processing: the influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients. https://doi.org/10.3390/nu9080835
Thornalley PJ, Langborg A, Minhas HS (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem J 344:109–116
Uribarri J et al (2003) Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Diseases 42:532–538. https://doi.org/10.1016/S0272-6386(03)00779-0
Uribarri J, Cai W, Sandu O, Peppa M, Goldberg T, Vlassara H (2005) Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann N Y Acad Sci 1043:461–466. https://doi.org/10.1196/annals.1333.052
Uribarri J et al (2010) Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc 110:911–916.e912. https://doi.org/10.1016/j.jada.2010.03.018
Van Boekel MAJS (1998) Effect of heating on Maillard reactions in milk. Food Chem 62:403–414. https://doi.org/10.1016/S0308-8146(98)00075-2
Wada Y, Lonnerdal B (2014) Effects of different industrial heating processes of milk on site-specific protein modifications and their relationship to in vitro and in vivo digestibility. J Agric Food Chem. https://doi.org/10.1021/jf501617s
Wolff SP, Dean RT (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem J 245:243–250
Acknowledgements
We kindly thank Michele Wölk for performing an experiment to exclude formyllysine formation during the sample preparation procedure. Financial support from the Deutsche Forschungsgemeinschaft (HO2222/7-1, INST 268/289-1) and the European Fund for Regional Structure Development (EFRE, European Union and Free State Saxony; 100055720, 100092961 and 100146238) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Handling Editor: F. Blachier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
726_2019_2707_MOESM1_ESM.docx
Supplementary material 1 (DOCX 9755 kb) Table S1 AGE-modified peptides and the corresponding modification sites. Table S2 AGE-modified milk proteins and their modification sites. Fig. S1 SDS-PAGE of milk proteins precipitated from commercial milk products. Fig. S2 SDS-PAGE of tryptic digests of milk proteins. Fig. S3 Strategy applied for the identification of AGEs in bovine milk proteins. Fig. S4 Structures of Lys- and Arg-derived AGEs. Fig. S5 CID and ETD tandem mass spectra of a CML-modified tryptic peptide. Fig. S6 CID and ETD tandem mass spectra of a pyralline-, FL and CML-modified tryptic peptides. Fig. S7 TICs and XICs of peptide 77 for a UHT-treated sample processed with formic acid or TFA. Fig. S8 Peak areas of peptides 7, 66, and 78 determined in a UHT-treated sample processed with formic acid or TFA. Fig. S9 Number of AGE-modified peptides derived from β-lactoglobulin. Fig. S10 Relative quantification of AGE-modified peptides of β-lactoglobulin
Rights and permissions
About this article
Cite this article
Milkovska-Stamenova, S., Hoffmann, R. Diversity of advanced glycation end products in the bovine milk proteome. Amino Acids 51, 891–901 (2019). https://doi.org/10.1007/s00726-019-02707-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-019-02707-4