Abstract
Recently, we reported a significant solvent effect on the phosphorus hyperfine coupling constant a P in β-phosphorylated 6-membered ring nitroxides (∆a P = 24 G in Org. Biomol. Chem. 2015). Thus, it led us to investigate the effect of solvent for several 6-membered ring nitroxides. Although smaller than mentioned above, a change of 5–7 G in a P with the polarity of solvent was still observed for these nitroxides. As for other β-phosphorylated nitroxides, a N increases with the polarity/polarizability π* and the Hydrogen Bond Donating α properties of the solvent whereas a P exhibits the reverse trends. The change of a P with the solvent depends a lot on a subtle interplay between the destabilizing steric hindrance due to the bulkiness of the substituents and the stabilizing hyperconjugation interactions SOMO → σ*C–P between the anti-bonding orbitals of the C–P bond and the SOMO.

















Similar content being viewed by others
Notes
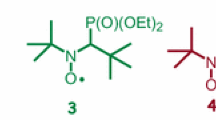
As far as we know, this nitroxide has only been observed through spin-trapping experiments involving the addition of 2-diethoxyphoshorylprop-2-yl radical onto tert-butyl nitroso. Consequently, its preparation on a large scale by a conventional procedure is not so obvious.
θ the dihedral angle between the C–P bond and the Singly Occupied Molecular Orbital SOMO (Fig. 2) on the nitrogen atom of the nitroxyl moiety. ρ πN , the electron density on the nitrogen atom of the nitroxyl moiety, is proportional to a N. B 0 is the transfer of the spin density through the spin polarization process and B 1 is the transfer of the spin density through the hyperconjugation process. In general, B 0 is very small and can be neglected. See Ref. [9]. Values of B 1 are dependent on the atom or on the group at position β See Refs. [9, 17].
CCDC: 1450111. The related X-ray parameters are provided in SI.
This conformation is the most stable as only one strong methyl-methyl 1,3-syn interaction is observed instead of 3 strong interactions in the conformer with t-BuMe2SiO and (EtO)2(O)P in axial positions (Me-t-BuMe2SiO, Me-(EtO)2(O)P and, t-BuMe2SiO-(EtO)2(O)P, not shown).
Many other parameters available in the literature can be used to describe the different terms of Eq. (18). Here, only the parameters used for the KP and KAT correlations are discussed.
As quoted by Reichardt (Ref. [12]): “A cybotactic region may be defined as the volume around a solute molecule in which the ordering of the solvent molecules has been influenced by the solute, including both the first solvation shell and the transition region”.
At the first glance, geometrical requirements for H-bonding are expected to be fulfilled for all nitroxides.
References
G. Likhtenshtein, J. Yamauchi, S. Nakatsuji, A.I. Smirnov, R. Tamura, Nitroxides: Applications in Chemistry, Biomedicine, and Materials Science (Wiley-VCH, Weinheim, 2008)
R. Hicks (ed.), Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds (Wiley, Hoboken, N.J., 2010)
A. Kirilyuk, A.A. Bobko, V.V. Khramtsov, I.A. Grigor’ev, Org. Biomol. Chem. 3, 1269–1274 (2005)
V. Belle, S. Rouger, S. Costanzo, S. Longhi, A. Fournel, in Instrumental Analysis of Intrinsically Disordered Proteins: Assessing Structures and Conformations, ed. by V.N. Uversky, S. Longhi (Wiley, Hoboken, N.J., 2010), pp. 131–169
P. Mellet, P. Massot, G. Madelin, S.R.A. Marque, E. Harte, J.-M. Franconi, E. Thiaudière, PLoS One. 4, e5244 (2009)
H. Karoui, F. Le Moigne, O. Ouari, P. Tordo, in Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, ed. by R. Hicks (Wiley, Hoboken, N.J., 2010), pp. 173–229
V. Ovcharenko, in Stable Radicals: Fundamentals and Applied Aspects of Odd-Electron Compounds, ed. by R. Hicks (Wiley, Hoboken, N.J., 2010), pp. 461–506
B.R. Knauer, J.J. Napier, J. Am. Chem. Soc. 98, 4395 (1976)
E.G. Janzen, G.A. Coulter, U.M. Oehler, J.P. Bergsma, Can. J. Chem. 60, 2725 (1982)
C.H. Deng, C.J. Guan, M.H. Shen, C.X. Zhao, J. Fluorine Chem. 116, 109 (2002)
A.Sh. Mukhtarov, A.V. Il’yasov, Ya.A. Levin, I.P. Gozman, M.S. Skorobogatova, E.I. Zoroatskaya, Theor. Exp. Chem. 12, 656 (1976); Teor. Eksp. Khim. 12, 831 (1976)
C. Reichardt, T. Welton, Solvent and Solvent Effect in Organic Chemistry, 4th ed. (Wiley-VCH, Weinheim, 2011)
G. Audran, L. Bosco, P. Brémond, J.-M. Franconi, N. Koonjoo, S.R.A. Marque, P. Massot, P. Mellet, E. Parzy, E. Thiaudière, Angew. Chem. Int. Ed., 54(45), 13379 (2015)
G. Audran, L. Bosco, P. Brémond, T. Butscher, S.R.A. Marque, Org. Biomol. Chem. 14, 1228 (2016)
G. Audran, L. Bosco, P. Brémond, T. Butscher, S.R.A. Marque, Appl. Magn. Reson., 45(12), 1333 (2015)
G. Audran, L. Bosco, P. Brémond, T. Butscher, J.-M. Franconi, K. Kabitaev, S.R.A. Marque, P. Mellet, E. Parzy, M. Santelli, E. Thiaudière, S. Viel, RSC Adv., 6, 5653–5670 (2016)
F. Gerson, W. Huber, Electron Spin Resonance Spectroscopy of Organic Radicals (Wiley-VCH, Weinheim, 2003)
S. Marque, J. Org. Chem. 68, 7582 (2003)
E.G. Bagryanskaya, S.R.A. Marque, Y.P. Tsentalovich, J. Org. Chem. 77, 4996 (2012)
E.G. Bagryanskaya. S.R.A. Marque, Chem. Rev. 114, 5011 (2014)
M. Charton, Prog. Phys. Org. Chem. 13, 119 (1981)
H. Fischer, A. Kramer, S.R.A. Marque, P. Nesvadba, Macromolecules 38, 9974 (2005)
G. Audran, P. Brémond, S.R.A. Marque, G. Obame, ChemPhysChem 13, 3542 (2012)
P. Stipa, J.-P. Finet, F. Le Moigne, P. Tordo, J. Org. Chem. 58, 4465 (1993)
Y. Marcus, The Properties of Solvents Vol. 4, (Wiley, Chichester, 1998)
G. E. Zaikov, R. G. Makitra, G. G. Midyana, L. I. Bazylyak, Influence of the Solvent on Some Radical Reaction, Chemistry Research and Applications Series (Nova Science Publishers Inc., New York, 2010)
J. Shorter, Correlation Analysis of Organic Reactivity (J. Wiley & Sons, New York, 1982), pp. 73–126
J. Shorter, Correlation Analysis of Organic Reactivity (J. Wiley & Sons, New York, 1982), pp. 9–25
A. Debuigne, D. Chan-Seng, L. Li, G.K. Hamer, M.K. Georges, Macromolecules 40, 6224 (2007)
R. Katritzky, M. Kuanar, S. Slavov, C.D. Hall, M. Karelson, I. Kahn, D.A. Dobchev, Chem. Rev. 110, 5714 (2010)
A. Koppel, V.A. Palm, in Advances in Linear Free Energy Relationships chapter 5, ed. by N.B. Chapman, J. Shorter (Plenum Press, London, New York, 1972), p. 203
G.A. Jeffrey, W. Saenger, Hydrogen Bonding in Biological Structures (Springer-Verlag, Berlin, Heidelberg, 1994)
R.S. Rowland, R. Taylor, J. Phys. Chem. 100, 7384 (1996)
Acknowledgements
We thank Aix-Marseille University, CNRS for financial support and Prof. Audran for advice in synthesis. SRAM thanks the Russian Science Foundation (project no. 15-13-20020) for supporting the correlation analysis. We thank RENARD network for the EPR platform.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bosco, L., Butscher, T. & Marque, S.R.A. β-Phosphorus Hyperfine Coupling Constant in Nitroxides: Conformational Effects in 6-Membered Ring Nitroxides. Appl Magn Reson 48, 379–406 (2017). https://doi.org/10.1007/s00723-017-0867-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-017-0867-z