Abstract
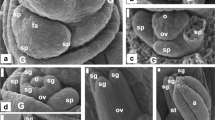
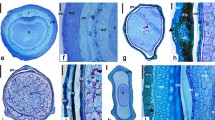
This is the first study to describe in a timescale morphohistological and ultrastructural characteristics of fruit (cypsela) and seed development in Trichocline catharinensis, which was completed 21 days after anthesis (DAA). At anthesis, we identified an ovary with three differentiated regions, including the inner epidermis, inner part, and outer epidermis. The mature ovule showed an integument with the outer epidermis, integumentary parenchyma, and endothelium. Cells around the endothelium form the periendothelial zone with thick cell walls that showed Periodic acid-Schiff (PAS)–positive reaction. The periendothelial zone and endothelium showed degradation of the cells during embryogenesis. The main stages of embryo development from fecundation through mature seed were identified. The ripe cypsela showed the pericarp (exocarp), seed coat (exotesta), and remaining endosperm surrounding the embryo. Mature embryos were straight with shoot apical meristem (SAM), and root apical meristem (RAM) was separated by the hypocotyl. Light microscopy (LM) and transmission electron microscopy (TEM) analyses indicate cells with characteristics of meristem cells, as well as proteins and lipid bodies and mitochondria with few cristae in cotyledon cells. Our findings provide insight into taxonomic and physiological studies by detailing cypsela and seed ontogenesis from an endemic and vulnerable Asteraceae from southern Brazil. This study is also a starting point for establishing the biological criteria for seed harvesting and future studies of seed physiology and conservation of plant genetic resource.






Similar content being viewed by others
Abbreviations
- CBB:
-
Coomassie brilliant blue (stain)
- DAA:
-
Day(s) after anthesis
- ER:
-
Endoplasmic reticulum
- LM:
-
Light microscopy
- lbs:
-
Lipid bodies
- PAS:
-
Periodic acid-Schiff (stain)
- pbs:
-
Protein bodies
- PCD:
-
Programmed cell death
- RAM:
-
Root apical meristem
- SAM:
-
Shoot apical meristem
- TB-O:
-
Toluidine blue (stain)
- TEM:
-
Transmission electron microscopy
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling
References
Aguado M, Martínez-Sánchez JJ, Reig-Arminana J, García-Breijo FJ, Franco JA, Vicente MJ (2011) Morphology, anatomy and germination response of heteromorphic achenes of Anthemis chrysantha J. Gay (Asteraceae), a critically endangered species. Seed Sci Res 21:283–294. https://doi.org/10.1017/S0960258511000183
Batista MF, da Silva Santos L, Muller RH, de Souza LA (2015) Seed characters and their usefulness in the separation of Asteraceae species. Acta Sci Biol Sci 37:505. https://doi.org/10.4025/actascibiolsci.v37i4.27964
Bouman F (1974) Developmental studies of the ovule, integuments and seed in some angiosperms. Dissertation, University of Amsterdam
Bradford KJ (2018) Interpreting biological variation: seeds, populations and sensitivity thresholds. Seed Sci Res 28(3):158–167
Cabrera AL, Klein RM (1973) Compostas tribo Mutisieae. In: Cabrera AL, Klein RM (eds) Flora ilustrada catarinense. Herbário Barbosa Rodrigues, Itajaí, p 94
Caccere R, Teixeira SP, Centeno DC, Rita de Cássia L, Braga MR (2013) Metabolic and structural changes during early maturation of Inga vera seeds are consistent with the lack of a desiccation phase. J Plant Physiol 170:791–800. https://doi.org/10.1016/j.jplph.2013.01.002
Chen B-X, Shi C-Y, Huang J-M, Wang M, Liu J-X (2014) Megasporogenesis, female gametophyte development and embryonic development of Ambrosia L. in China. Plant Syst Evol 300:197–208. https://doi.org/10.1007/s00606-013-0872-0
Colette A, Lu K-J, Weijers D (2015) Building a plant: cell fate specification in the early Arabidopsis embryo. Development 142:420–430. https://doi.org/10.1242/dev.111500
de Oliveira Franca R, De-Paula OC, Carmo-Oliveira R, Marzinek J (2015) Embryology of Ageratum conyzoides L. and A. fastigiatum RM King & H. Rob.(Asteraceae). Acta Bot Bras 29:08–15. https://doi.org/10.1590/0102-33062014abb3609
Dinneny JR, Yanofsky MF (2005) Drawing lines and borders: how the dehiscent fruit of Arabidopsis is patterned. Bioessays 27:42–49. https://doi.org/10.1002/bies.20165
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
dos Santos Julio PG, Oliveira DMT (2009) Morfoanatomia comparada e ontogênese do pericarpo de Bidens gardneri Baker e B. pilosa L.(Asteraceae). Braz J Bot 32:109–116. https://doi.org/10.1590/S0100-84042009000100011
Farrant JM, Pammenter N, Berjak P, Walters C (1997) Subcellular organization and metabolic activity during the development of seeds that attain different levels of desiccation tolerance. Seed Sci Res 7:135–144. https://doi.org/10.1017/S0960258500003470
Figueiredo R, Duarte P, Pereira S, Pissarra J (2006) The embryo sac of Cynara cardunculus: ultrastructure of the development and localisation of the aspartic proteinase cardosin B. Sex Plant Reprod 19:93–101. https://doi.org/10.1007/s00497-006-0026-4
Frangiote-Pallone S, de Souza LA (2014) Pappus and cypsela ontogeny in Asteraceae: structural considerations of the tribal category. Revista Mexicana de Biodiversidad 85:62–77. https://doi.org/10.7550/rmb.32809
Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G (2003) Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature 426:147–153. https://doi.org/10.1038/nature02085
Funk VA, Susanna A, Stuessy TF, Robinson H (2009) Classification of Compositae. In: Funk VA, Susanna A, Stuessy T, Bayer R (eds) Systematics, evolution and biogeography of the Compositae, pp 171–189
Gahan PB (1984) Plant histochemistry and cytochemistry. Academic Press
Galastri NA, Oliveira DMTD (2010) Morfoanatomia e ontogênese do fruto e semente de Vernonia platensis (Spreng.) Less.(Asteraceae). Acta Bot Bras 24:73–83. https://doi.org/10.1590/S0102-33062010000100008
Goldberg RB, De Paiva G, Yadegari R (1994) Plant embryogenesis: zygote to seed. Science 266:605–614. https://doi.org/10.1126/science.266.5185.605
Gordon E, McCandless E (1973) Ultrastructute and histochemistry of Chondrus crispus Stackhouse. Nova Scotian Inst Sci Proc
Gotelli MM, Galati BG, Medan D (2008) Embryology of Helianthus annuus (Asteraceae). In: Ann Bot Fenn, vol 2. BioOne, pp 81–96
Greenwood JS, Helm M, Gietl C (2005) Ricinosomes and endosperm transfer cell structure in programmed cell death of the nucellus during Ricinus seed development. Proc Natl Acad Sci 102:2238–2243
Haywood V, Kragler F, Lucas WJ (2002) Plasmodesmata: pathways for protein and ribonucleoprotein signaling. Plant Cell 14:S303–S325
Jana BK, Mukherjee SK (2014) Diversity of cypselar features and their taxonomic significance in three species of the genus Centaurea, tribe Cardueae of Asteraceae. Diversity and conservation of plants and traditional knowledge, pp 233–240
Jenik PD, Gillmor CS, Lukowitz W (2007) Embryonic patterning in Arabidopsis thaliana. Annu Rev Cell Dev Biol 23:207–236. https://doi.org/10.1146/annurev.cellbio.22.011105.102609
Jensen WA (1962) Botanical Histochemistry. Principles and practices. Freeman, San Francisco
Jimenez-Lopez JC, Zienkiewicz A, Zienkiewicz K, Alché JD, Rodríguez-García MI (2016) Biogenesis of protein bodies during legumin accumulation in developing olive (Olea europaea L.) seed. Protoplasma 253:517–530. https://doi.org/10.1007/s00709-015-0830-5
Johansen DA (1940) Plant microtechnic. Mc Grow Hill Book Company, Inc, New York
Jones L, Milne JL, Ashford D, McQueen-Mason SJ (2003) Cell wall arabinan is essential for guard cell function. Proc Natl Acad Sci 100:11783–11788. https://doi.org/10.1073/pnas.1832434100
Jouannic S, Lartaud M, Hervé J, Collin M, Orieux Y, Verdeil JL, Tregear JW (2011) The shoot apical meristem of oil palm (Elaeis guineensis ; Arecaceae): developmental progression and dynamics. Ann Bot 108:1477–1487. https://doi.org/10.1093/aob/mcr019
Jürgens G (2001) Apical–basal pattern formation in Arabidopsis embryogenesis. EMBO J 20:3609–3616. https://doi.org/10.1093/emboj/20.14.3609
Kapil RN, Tiwari SC (1978) The integumentary tapetum. Bot Rev 44:457-490. https://doi.org/10.1007/bf02860847
Katinas L, Pasini E (2016) Typifications in the genus Trichocline (Asteraceae: Mutisieae). Willdenowia 46:27–35
Katinas L, Pruski J, Sancho G, Tellería MC (2008) The subfamily Mutisioideae (Asteraceae). Bot Rev 74:469–716
Kermode AR (2011) Plant storage products (carbohydrates, oils and proteins). eLS
Kolczyk J, Stolarczyk P, Płachno BJ (2014) Comparative anatomy of ovules in Galinsoga, Solidago and Ratibida (Asteraceae). Acta Biol Cracov Ser Bot 56:115–125. https://doi.org/10.2478/abcsb-2014-0024
Koltunow A, Johnson SD, Bicknell RA (1998) Sexual and apomictic development in Hieracium. Sex Plant Reprod 11:213–230
Leprince O, Hendry G, McKersie B (1993) The mechanisms of desiccation tolerance in developing seeds. Seed Sci Res 3:231–246
Leprince O, Pellizzaro A, Berriri S, Buitink J (2017) Late seed maturation: drying without dying. J Exp Bot 68:827–841
Long RL, Gorecki MJ, Renton M, Scott JK, Colville L, Goggin DE, Commander LE, Westcott DA, Cherry H, Finch-Savage WE (2015) The ecophysiology of seed persistence: a mechanistic view of the journey to germination or demise. Biol Rev 90:31–59
Maheswari Devi H (1957) Embryological studies in Compositae. Proc Plant Sci 46:68–74. https://doi.org/10.1007/BF03052108
Manning J, Simka B, Boatwright J, Magee A (2016) A revised taxonomy of Gerbera sect. Gerbera (Asteraceae: Mutisieae). S Afr J Bot 104:142–157
Martins MAG, Oliveira DMT (2007) Morfoanatomia comparada dos frutos em desenvolvimento de Vernonia brevifolia Less. e V. herbacea (Vell.) Rusby (Asteraceae). Braz J Bot:101–112
Marzinek J, Oliveira DM (2010) Structure and ontogeny of the pericarp of six Eupatorieae (Asteraceae) with ecological and taxonomic considerations. An Acad Bras Cienc 82:279–291
Marzinek J, De-Paula OC, Oliveira DMT (2008) Cypsela or achene? Refining terminology by considering anatomical and historical factors. Braz J Bot 31:549–553
McCartney L, Ormerod AP, Gidley MJ, Knox JP (2000) Temporal and spatial regulation of pectic (1→ 4)-β-D-galactan in cell walls of developing pea cotyledons: implications for mechanical properties. Plant J 22:105–113
Moore JP, Vicré-Gibouin M, Farrant JM, Driouich A (2008) Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol Plant 134:237–245
Moura EF, Ventrella MC, Motoike SY (2010) Anatomy, histochemistry and ultrastructure of seed and somatic embryo of Acrocomia aculeata (Arecaceae). Sci Agric 67:399–407
Müntz K (1998) Deposition of storage proteins. In: Protein trafficking in plant cells, Springer, pp 77–99
Musiał K, Kościńska-Pająk M (2013) Ovules anatomy of selected apomictic taxa from Asteraceae family. Mod Phytomorphol 3:35–38
Musiał K, Kościńska-Pająk M, Sliwinska E, Joachimiak AJ (2012) Developmental events in ovules of the ornamental plant Rudbeckia bicolor Nutt. Flora-Morphol Distrib Funct Ecol Plants 207:3–9. https://doi.org/10.1016/j.flora.2011.07.015
Musiał K, Płachno B, Świątek P, Marciniuk J (2013) Anatomy of ovary and ovule in dandelions (Taraxacum, Asteraceae). Protoplasma 250:715–722
Nakajima K, Benfey PN (2002) Signaling in and out: control of cell division and differentiation in the shoot and root. Plant Cell 14:S265–S276
Nakajima JN, Loeuille B, Heiden G, Dematteis, M, Hattori EKO, Magenta MAG, Ritter MR, Mondin CA, Roque N, Ferreira SC, Borges RA, Soares PN, Almeida G, Schneider A, Sancho G, Saavedra MM, Liro, RM, Pereira ACM, Moraes MD, Silva GAR, Medeiros JD, Lorencini TS, Teles AM, Monge M, Siniscalchi CM, Souza-Buturi FO, Bringel JR JBA, Carneiro CR, Pasini E, Oliveira CT (2016) Asteraceae in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available in: . Accessed 20 Jan 2016
Nieuwland J, Scofield S, Murray JAH (2009) Control of division and differentiation of plant stem cells and their derivatives. Semin Cell Dev Biol 20:1134–1142
Pasini E, Ritter MR (2012) O gênero Trichocline Cass.(Asteraceae, Mutisieae) no Rio Grande do Sul, Brasil. Revista Brasileira de Biociências 10
Płachno BJ, Świątek P, Kozieradzka-Kiszkurno M, Majeský Ľ, Marciniuk J, Stolarczyk P (2015) Are obligatory apomicts invested in the pollen tube transmitting tissue? Comparison of the micropyle ultrastructure between sexual and apomictic dandelions (Asteraceae, Lactuceae). Protoplasma 252:1325–1333. https://doi.org/10.1007/s00709-015-0765-x
Płachno BJ, Kurczyńska E, Świątek P (2016) Integument cell differentiation in dandelions (Taraxacum, Asteraceae, Lactuceae) with special attention paid to plasmodesmata. Protoplasma 253:1365–1372. https://doi.org/10.1007/s00709-015-0894-2
Probert RJ, Daws MI, Hay FR (2009) Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Ann Bot 104:57–69
Pullaiah T (1981) Studies in the embryology ofHeliantheae (Compositae). Plant Syst Evol 137:203–214
Rogge-Renner GD, Steiner N, Schmidt ÉC, Bouzon ZL, Farias FL, Guerra MP (2013) Structural and component characterization of meristem cells in Araucaria angustifolia (Bert.) O. Kuntze zygotic embryo. Protoplasma 250:731–739. https://doi.org/10.1007/s00709-012-0457-8
Rudall PJ (1997) The nucellus and chalaza in monocotyledons: structure and systematics. Bot Rev 63:140–181. https://doi.org/10.1007/BF02935930
Schmidt EC, Scariot LA, Rover T, Bouzon ZL (2009) Changes in ultrastructure and histochemistry of two red macroalgae strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a consequence of ultraviolet B radiation exposure. Micron 40:860–869
Schmidt ÉC, Dos Santos R, Horta PA, Maraschin M, Bouzon ZL (2010) Effects of UVB radiation on the agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): changes in cell organization, growth and photosynthetic performance. Micron 41:919–930. https://doi.org/10.1016/j.micron.2010.07.010
Schmidt ÉC, Pereira B, dos Santos RW, Gouveia C, Costa GB, Faria GSM, Scherner F, Horta PA, Martins RP, Latini A, Ramlov F, Maraschin M, Bouzon ZL (2012) Responses of the macroalgae Hypnea musciformis after in vitro exposure to UV-B. Aquat Bot 100:8–17. https://doi.org/10.1016/j.aquabot.2012.03.004
Singh MB, Bhalla PL (2006) Plant stem cells carve their own niche. Trends Plant Sci 11:241–246. https://doi.org/10.1016/j.tplants.2006.03.004
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Steiner N et al (2015) Toward establishing a morphological and ultrastructural characterization of proembryogenic masses and early somatic embryos of Araucaria angustifolia (Bert.) O. Kuntze. Protoplasma 253:487-501. https://doi.org/10.1007/s00709-015-0827-0
Sul RGD (2014) Decreto n. ° 52.109, de 02 de dezembro de 2014 vol 233
Tan-Wilson AL, Wilson KA (2012) Mobilization of seed protein reserves. Physiol Plant 145:140–153. https://doi.org/10.1111/j.1399-3054.2011.01535.x
van Baarlen P, Verduijn M, van Dijk PJ (1999) What can we learn from natural apomicts? Trends Plant Sci 4:43–44
Veloso VHS, Ribeiro LM, Mercadante-Simões MO, Nunes YRF (2016) Cytological aspects of recalcitrance in dormant seeds of Mauritia flexuosa (Arecaceae). Acta Physiol Plant 38:171. https://doi.org/10.1007/s11738-016-2194-7
Verdeil J-L, Alemanno L, Niemenak N, Tranbarger TJ (2007) Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends Plant Sci 12:245–252
von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Weigel D, Jürgens G (2002) Stem cells that make stems. Nature 415:751–754
Werker E (1997) Seed anatomy. Gebruder Borntraeger Verlagsbuchhandlung
Wesley-Smith J, Walters C, Pammenter N, Berjak P (2015) Why is intracellular ice lethal? A microscopical study showing evidence of programmed cell death in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum. Ann Bot 115:991–1000. https://doi.org/10.1093/aob/mcv009
West M, Harada JJ (1993) Embryogenesis in higher plants: an overview. Plant Cell 5:1361–1369
Western TL (2012) The sticky tale of seed coat mucilages: production, genetics, and role in seed germination and dispersal. Seed Sci Res 22:1–25
Yurukova-Grancharova P, Robeva-Davidova P, Vladimirov V (2006) On the embryology and mode of reproduction of selected diploid species of Hieracium sl (Asteraceae) from Bulgaria. Flora-Morphol Distrib Funct Ecol Plants 201:668–675. https://doi.org/10.1016/j.flora.2006.01.003
Zanin A, Longhi-Wagner HM, Rieper M (2010) Fitofisionomia das formações campestres do Campo dos Padres, Santa Catarina, Brasil. INSULA Revista de Botânica 38:42
Zhang Z, Laux T (2011) The asymmetric division of the Arabidopsis zygote: from cell polarity to an embryo axis. Sex Plant Reprod 24:161–169. https://doi.org/10.1007/s00497-010-0160-x
Acknowledgments
The authors acknowledge the staff of the Central Laboratory of Electron Microscopy (LCME) and Plant Cell Biology Laboratory of the Federal University of Santa Catarina, Florianópolis, Santa Catarina, Brazil.
Funding
This work was supported by the National Council for Scientific and Technological Development (CNPq, Brazil), Proc. No. (311156/2017-7 457940/2014-0). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) -Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Elias, R.A., Lando, A.P., Viana, W.G. et al. Structural aspects of cypsela and seed development of Trichocline catharinensis (Cabrera): a Brazilian endemic species. Protoplasma 256, 1495–1506 (2019). https://doi.org/10.1007/s00709-019-01361-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-019-01361-7