Abstract
The experimental models of the development of cirrhosis in rats require a long time. Many studies in animals have demonstrated similarities in histological pattern with human cirrhosis. Just like the relation between cirrhosis and increased lipid peroxidation (LPO), which contributes to the worsening of the disease. However, few studies have focused on the reduction of time to establish cirrhosis and evaluated the expression of heat-shock protein 70 (HSP70) in cirrhotic livers of rodents. The present study proposes the adaptation of an experimental cirrhosis model using diethylnitrosamine (DEN). Twenty-six male Wistar rats, weighing ±270 g, divided into two groups: (i) CO-control and (ii) DEN-diethylnitrosamine. The DEN group received 50 mg/kg of DEN twice a week intraperitoneally for 7 weeks. The model developed cirrhosis in 7 weeks. The liver function tests showed that the animals with DEN-induced cirrhosis had increased levels when compared to control. The histological examination showed changes in the liver architecture, with severe ductal proliferation, signs of chronic damage, cholestasis, lymphocytic infiltrate, steatosis, and extensive parenchymal loss. We also found nodular formations with homogeneous pattern, increased LPO, increased expression of iNOS, TGF beta, α-SMA, and NQO1. However, the HSP70 expression was reduced in cirrhotic animals. This study showed signs of cirrhosis in liver based on biochemical, histological, and molecular analysis. The reduced expression of HSP70 appears to be associated with increased oxidative stress, contributing to the worsening of the disease.





Similar content being viewed by others
References
Adachi H, Katsuno M, Waza M, Minamiyama M, Tanaka F, Sobue G (2009) Heat shock proteins in neurodegenerative diseases: pathogenic roles and therapeutic implications. Int J Hyperthermia 25:647–654
Aleksunes LM, Goedken M, Manautou JE (2006) Up-regulation of NAD(P)H quinone oxidoreductase 1 during human liver injury. World J Gastroenterol 12:1937–1940
Altrock E, Sens C, Wuerfel C, Vasel M, Kawelke N, Dooley S, Sottile J et al (2014) Inhibition of fibronectin deposition improves experimental liver fibrosis. J Hepatol
AVMA (2007) AVMA Guidelines on Euthanasia (Formerly Report of the AVMA Panel on Euthanasia). pp 1–39
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 15:209–218
Bona S, Filippin LI, Di Naso FC, de David C, Valiatti B, Isoppo Schaun M, Xavier RM, Marroni NP (2012) Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride-induced cirrhosis. ISRN Gastroenterol 2012:1–7
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brenner C, Galluzzi L, Kepp O, Kroemer G (2013) Decoding cell death signals in liver inflammation. J Hepatol 59:583–594
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Cameron J, Karunarete W (1936) Tetrachloride cirrhosis in relation to liver regeneration. J Pathol Bacteriol 42:1–21
Corpechot C, Barbu V, Wendum D, Chignard N, Housset C, Poupon R, Rosmorduc O (2002) Hepatocyte growth factor and c-Met inhibition by hepatic cell hypoxia: a potential mechanism for liver regeneration failure in experimental cirrhosis. Am J Pathol 160:613–620
Cremonese RV, Pereira-Filho AA, Magalhães R, de Mattos AA, Marroni CA, Zettler CG, Marroni NP (2001) Experimental cirrhosis induced by carbon tetrachloride inhalation: adaptation of the technique and evaluation of lipid peroxidation. Arq Gastroenterol 38:40–47
Friedman SL (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134:1655–1669
Gomer CJ, Ryter SW, Ferrario A, Rucker N, Wong S, Fisher AM (1996) Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res 56:2355–2360
González-Ramos M, Calleros L, López-Ongil S, Raoch V, Griera M, Rodríguez-Puyol M, de Frutos S, Rodríguez-Puyol D (2013) HSP70 increases extracellular matrix production by human vascular smooth muscle through TGF-β1 up-regulation. Int J Biochem Cell Biol 45:232–242
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S (2002) Roles of TGF-beta in hepatic fibrosis. Front Biosci 7:d793–d807
Gressner OA, Weiskirchen R, Gressner AM (2007) Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin Chim Acta 381:107–113
Guo S, Wharton W, Moseley P, Shi H (2007) Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 12:245–254
Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A (2010) HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol 8:1–15
Haratake J, Hisaoka M, Yamamoto O, Horie A (1991) Morphological changes of hepatic microcirculation in experimental rat cirrhosis: a scanning electron microscopic study. Hepatology 13:952–956
Hooper PL, Hooper JJ (2005) Loss of defense against stress: diabetes and heat shock proteins. Diabetes Technol Ther 7:204–208
Imaoka S, Osada M, Minamiyama Y, Yukimura T, Toyokuni S, Takemura S, Hiroi T, Funae Y (2004) Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett 203:117–125
Jacquier-Sarlin MR, Polla BS (1996) Dual regulation of heat-shock transcription factor (HSF) activation and DNA-binding activity by H2O2: role of thioredoxin. Biochem J 318(Pt 1):187–193
Jacquier-Sarlin MR, Jornot L, Polla BS (1995) Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. J Biol Chem 270:14094–14099
Jiménez W, Clária J, Arroyo V, Rodés J (1992) Carbon tetrachloride induced cirrhosis in rats: a useful tool for investigating the pathogenesis of ascites in chronic liver disease. J Gastroenterol Hepatol 7:90–97
Krause M, Rodrigues-Krause JC (2011) Extracellular heat shock proteins (eHSP70) in exercise: possible targets outside the immune system and their role for neurodegenerative disorders treatment. Med Hypotheses 76:286–290
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li Z, Chen X, Meng J, Deng L, Ma H, Csete M, Xiong L (2012) Ed50 and recovery times after propofol in rats with graded cirrhosis. Anesth Analg 114:117–121
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677
Liu RM, Gaston Pravia KA (2010) Oxidative stress and glutathione in TGF-beta-mediated fibrogenesis. Free Radic Biol Med 48:1–15
Malik S, Bhatnagar S, Chaudhary N, Katare DP, Jain SK (2013) DEN + 2-AAF-induced multistep hepatotumorigenesis in Wistar rats: supportive evidence and insights. Protoplasma 250:175–183
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17:235–248
Mikami K, Otaka M, Goto T, Miura K, Ohshima S, Yoneyama K et al (2004) Induction of a 72-kda heat shock protein and protection against lipopolysaccharide-induced liver injury in cirrhotic rats. J Gastroenterol Hepatol 19:884–890
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Morimoto RI, Kroeger PE, Cotto JJ (1996) The transcriptional regulation of heat shock genes: a plethora of heat shock factors and regulatory conditions. EXS 77:139–163
Nciri R, Allagui MS, Bourogaa E, Saoudi M, Murat JC, Croute F, Elfeki A (2012) Lipid peroxidation, antioxidant activities and stress protein (HSP72/73, GRP94) expression in kidney and liver of rats under lithium treatment. J Physiol Biochem 68:11–18
Rodrigues-Krause J, Krause M, O’Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G (2012) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17:293–302
Ross D (2004) Quinone reductases multitasking in the metabolic world. Drug Metab Rev 36:639–654
Sarge KD, Murphy SP, Morimoto RI (1993) Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol Cell Biol 13:1392–1407
Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D (1997) The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol 52:300–305
Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D (2004) NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 65:1238–1247
Sturgill MG, Lambert GH (1997) Xenobiotic-induced hepatotoxicity: mechanisms of liver injury and methods of monitoring hepatic function. Clin Chem 43:1512–1526
Swenberg JA, Hoel DG, Magee PN (1991) Mechanistic and statistical insight into the large carcinogenesis bioassays on N-nitrosodiethylamine and N-nitrosodimethylamine. Cancer Res 51:6409–6414
Teufelhofer O, Parzefall W, Kainzbauer E, Ferk F, Freiler C, Knasmüller S, Elbling L, Thurman R, Schulte-Hermann R (2005) Superoxide generation from Kupffer cells contributes to hepatocarcinogenesis: studies on NADPH oxidase knockout mice. Carcinogenesis 26:319–329
Tieppo J, Cuevas MJ, Vercelino R, Tuñón MJ, Marroni NP, González-Gallego J (2009) Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. J Nutr 139:1339–1346
Tuñón MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Alvarez M et al (2013) Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res 55:221–228
Venugopal R, Jaiswal AK (1996) Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A 93:14960–14965
Verma IM, Stevenson J (1997) IkappaB kinase: beginning, not the end. Proc Natl Acad Sci U S A 94:11758–11760
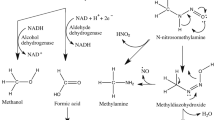
Verna L, Whysner J, Williams GM (1996) N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 71:57–81
Wasser S, Lim GY, Ong CN, Tan CE (2001) Anti-oxidant ebselen causes the resolution of experimentally induced hepatic fibrosis in rats. J Gastroenterol Hepatol 16:1244–1253
Watanabe D, Otaka M, Mikami K, Yoneyama K, Goto T, Miura K et al (2004) Expression of a 72-kda heat shock protein, and its cytoprotective function, in gastric mucosa in cirrhotic rats. J Gastroenterol 39:724–733
Yan LJ, Christians ES, Liu L, Xiao X, Sohal RS, Benjamin IJ (2002) Mouse heat shock transcription factor 1 deficiency alters cardiac redox homeostasis and increases mitochondrial oxidative damage. EMBO J 21:5164–5172
Acknowledgments
This study was supported by grants from the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo de Incentivo à Pesquisa e Eventos (FIPE) of the Hospital de Clínicas of Porto Alegre (HCPA), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), Laboratório Experimental de Hepatologia e Gastroenterologia (HCPA/UFRGS), and Laboratório de Estresse Oxidativo e Antioxidantes (ULBRA).
Conflict of interest
The authors of this article declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Reimer Stick
Rights and permissions
About this article
Cite this article
Bona, S., Moreira, A.J., Rodrigues, G.R. et al. Diethylnitrosamine-induced cirrhosis in Wistar rats: an experimental feasibility study. Protoplasma 252, 825–833 (2015). https://doi.org/10.1007/s00709-014-0719-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0719-8