Abstract
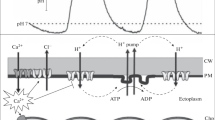
The charging of the plasma membrane is a necessary condition for the generation of an electric-field-induced permeability increase of the plasmalemma, which is usually explained by the creation and the growth of aqueous pores. For cells suspended in physiological buffers, the time domain of membrane charging is in the submicrosecond range. Systematic measurements using Nicotiana tabacum L. cv. Bright Yellow 2 (BY-2) protoplasts stained with the fast voltage-sensitive fluorescence dye ANNINE-6 have been performed using a pulsed laser fluorescence microscopy setup with a time resolution of 5 ns. A clear saturation of the membrane voltage could be measured, caused by a strong membrane permeability increase, commonly explained by enhanced pore formation, which prevents further membrane charging by external electric field exposure. The field strength dependence of the protoplast’s transmembrane potential V M shows strong asymmetric saturation characteristics due to the high resting potential of the plants plasmalemma. At the pole of the hyperpolarized hemisphere of the cell, saturation starts at an external field strength of 0.3 kV/cm, resulting in a measured transmembrane voltage shift of ∆V M = −150 mV, while on the cathodic (depolarized) cell pole, the threshold for enhanced pore formation is reached at a field strength of approximately 1.0 kV/cm and ∆V M = 450 mV, respectively. From this asymmetry of the measured maximum membrane voltage shifts, the resting potential of BY-2 protoplasts at the given experimental conditions can be determined to V R = −150 mV. Consequently, a strong membrane permeability increase occurs when the membrane voltage diverges |V M| = 300 mV from the resting potential of the protoplast. The largest membrane voltage change at a given external electric field occurs at the cell poles. The azimuthal dependence of the transmembrane potential, measured in angular intervals of 10° along the circumference of the cell, shows a flattening and a slight decrease at higher fields at the pole region due to enhanced pore formation. Additionally, at the hyperpolarized cell pole, a polarization reversal could be observed at an external field range around 1.0 kV/cm. This behavior might be attributed to a fast charge transfer through the membrane at the hyperpolarized pole, e.g., by voltage-gated channels.




Similar content being viewed by others
References
Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, Schoenbach KH (2002) Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: apoptosis induction and tumor growth inhibition. IEEE Trans Plasma Sci 30:286–292
Beebe SJ, Fox PM, Rec LJ, Willis EL, Schoenbach KH (2003) Nanosecond high-intensity pulsed electric fields induce apoptosis in human cells. FASEB J 17:1493–1495
Bezanilla F (2005) Voltage-gated ion channels. IEEE Trans Nanobiosci 4:1
Böckmann RA, de Groot BL, Kakorin S, Neumann E, Grubmüller H (2008) Kinetics, statistics, and energetics of lipid membrane electroporation studied by molecular dynamics simulations. Biophys J 95:1837–1850
Buescher ES, Schoenbach KH (2003) Effects of submicrosecond, high intensity pulsed electric fields on living cells-intracellular electromanipulation. IEEE Trans Dielectr Electr Insul 10:788–794
Chen C, Smye SW, Robinson MP, Evans JA (2006) Membrane electroporation theories: a review. Med Biol Eng Comput 44:5–14
Cole KS (1928) Electric impedance of suspensions of spheres. J Gen Physiol 12:29–36
Cole KS (1972) Membranes, ions and impulses. University of California Press, Berkeley, p 569
Doyle DA (2004) Structural changes during ion channel gating. Trends Neurosci 27:6
Frey W, Baumung K, Kolb JF, Chen N, White J, Morrison MA, Beebe SJ, Schoenbach KH (2004) Real-time imaging of the membrane charging of mammalian cells exposed to nanosecond pulsed electric fields. Conference Record of the 26th International Power Modulator Symposium pp 216–219
Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nuccitelli R, Beebe SJ, Schoenbach KH, Kolb JF (2006) Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophys J 90:3608–3615
Fromherz P (1995) Monopole–dipole model for symmetrical solvatochromism of hemicyanine dyes. J Phys Chem 99:7188–7192
Fromm J, Lautner S (2007) Electrical signals and their physiological significance in plants. Plant Cell Environ 30:249–257
Gabriel B, Teissie J (1997) Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys J 73:2630–2637
Gelli A, Blumwald E (1997) Hyperpolarization-activated Ca2+-permeable channels in the plasma membrane of tomato cells. J Mem Biol 155:34–45
Hibino M, Shigemori M, Itoh H, Nagayama K, Kinosita K (1991) Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J 59:209–220
Hu Q, Shridhara V, Joshi RP, Kolb JF, Schoenbach KH (2006) Molecular dynamics analysis of high electric pulse effects on bilayer membranes containing DPPC and DPPS. IEEE Trans Plasma Sci 34:4
Huang Y, Sekhon NS, Borninski J, Chen N, Rubinsky B (2003) Instantaneous, quantitative single cell viability assessment by electrical evaluation of cell membrane integrity with microfabricated devices. Sensors Actuat A 105:31–39
Hübner G, Lambacher A, Fromherz P (2003) Anellated hemicyanine dyes with large symmetrical solvatochromism of absorption and fluorescence. J Phys Chem 107:7896–7902
Kiegle E, Gilliham M, Haselhoff J, Tester M (2000) Hyperpolarisation-activated calcium currents found only on cells from the elongation zone of Arabidopsis thaliana roots. Plant J 21(2):225–229
Krassowska W, Filev PD (2007) Modeling electroporation in a single cell. Biophys J 92:404–417
Kuhn B, Fromherz P (2003) Annelated hemicyanine dyes in a neuron membrane: molecular stark effect and optical voltage recording. Phys Chem 107:7903–7913
Kuhn B, Fromherz P, Denk W (2004) High sensitivity of Stark-shift voltage-sensing dyes by one- or two-photon excitation near the red spectral edge. Biophys J 87:631–639
Maconochie DJ, Fletcher GH, Steinbach JH (1995) The conductance of the muscle nicotinic receptor channel changes rapidly upon Gatin. Biophys J 68:483–490
Mason E (1999) Potentiometric membrane dyes and imaging membrane potential in single cells. In fluorescent and luminescent probes for biological activity. Academic, New York, pp 210–221
Mehrle W, Hampp R, Zimmermann U (1989) Electric pulse induced membrane permeabilisation spatial orientation and kinetics of solute efflux in freely suspended and dielectrophoretically aligned plant mesophyll protoplasts. Biochim Biophys Acta 978:267–275
Melikov KC, Frolov VA, Ahcherbakov A, Samsoov AV, Chizmadzhev YA (2001) Voltage-induced nonconductive and metastable pores in unmodified lipid bilayers. Biophys J 80:1829–1836
Mongrand S, Morel J, Laroche J, Claverol S, Carde J-P, Hartmann M-A, Bonneu M, Simon-Plas F, Lessire R, Bessoule J-J (2004) Lipid rafts in higher plant cells. J Biol Chem 279:36277–36286
Neu JC, Krassowska W (2003) Modeling postshock evolution of large electropores. Physical Review E 67:1–12
Pakhomov AG, Bowman AM, Ibey BL, Andre FM, Pakhomova ON, Schoenbach KH (2009) Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem Biophys Res Commun 385:181–186
Pauly H, Schwan HP (1959) Über die Impedanz einer Suspension von kugelförmigen Teilchen mit einer Schale, Z. Naturforschung 14b:125–131
Phez E, Faurie C, Golzio M, Teissié J, Rols MP (2005) New insights in the visualization of membrane permeabilization and DNA/membrane interaction of cells submitted to electric pulses. Biochim Biophys Acta 1724:248–254
Pliquett U, Joshi RP, Shridhara V, Schoenbach KH (2007) High electrical field effects on cell membranes. Bioelectrochemistry 70:275–282
Rieder A, Schwartz T, Schön-Hölz K, Marten SM, Süß J, Gusbeth C, Kohnen W, Swoboda W, Obst U, Frey W (2008) Molecular monitoring of inactivation efficiencies of bacteria during pulsed electric field (PEF) treatment of clinical wastewater. J Appl Microbiol 105:2035–2045
Sack M, Bluhm H (2006) Electroporation of slices of sugar beets with rectangular pulses. Plasma Science, ICOPS 2006, IEEE Conference Record – Abstracts pp 438-438
Sack M, Schultheiss C, Bluhm H (2005) Triggered Marx generators for the industrial-scale electroporation of sugar beets. IEEE Trans Ind Appl 41(3):707–714
Sack M, Eing C, Buth L, Berghöfer T, Frey W, Bluhm H (2007) Electroporation as an optimizing step in the drying of green biomass. Plasma Science, IEEE Conference Record—Abstracts pp 518–518
Sack M, Eing C, Stängle R, Wolf A, Müller G, Sigler J, Stukenbrock L (2008) On the electroporation of mash for the production of red wine. ICOPS 2008, Proceedings of the IEEE 35th International Conference on Plasma Science
Scarlett SS, White JA, Blackmore PF, Schoenbach KH, Kolb JF (2009) Regulation of intracellular calcium concentration by nanosecond pulsed electric fields. Biochimica et Biophysica Acta 1788:1168–1175
Smith KC, Weaver JC (2008) Active mechanisms are needed to describe cell responses to submicrosecond, megavolt-per-meter pulses: cell models for ultrashort pulses. Biophys J 95:1547–1563
Sukhorukov VL, Endter JM, Zimmermann D, Shirakashi R, Fehrmann S, Kiesel M, Reuss R, Becker D, Hedrich R, Bamberg E, Roitsch Th, Zimmermann U (2007) Mechanisms of electrically mediated cytosolic Ca21 transients in aequorin-transformed tobacco cells. Biophys J 93:3324–3337
Teissie J, Rols MP (1993) An experimental evaluation of the critical potential difference inducing cell membrane electropermeabilization. Biophys J 65:409–413
Tekle E, Astumian RD, Chock PB (1990) Electropermeabilization of cell membranes: effect of the resting membrane potential. Biochem Biophys Res Commun 172:282–287
Tekle E, Astumian RD, Chock PB (1994) Selective and asymmetric molecular transport across electroporated cell membranes. Proc Natl Acad Sci USA 91:11512–11516
Vernier PT, Sun YH, Marcu L, Craft CM, Gundersen MA (2004) Nanosecond pulsed electric fields perturb membrane phospholipids in T-lymphoblasts. FEBS Lett 572:103–108
Weaver JC, Chizmadzhev YA (1996) Theory of electroporation: a review. Bioelectrochem Bioenerg 41:135–160
White PJ (2000) Calcium channels in higher plants. Biochim Biophys Acta 1465:171–189
White JA, Blackmore PF, Schoenbach KH, Beebe SJ (2004) Stimulation of capacitive calcium entry in HL-60 cells by nanosecond pulsed electric fields. J Biol Chem 279:22964–22972
Yang Y, Mayer LM, Wickremasinghe NS, Hafner JH (2008) Probing the lipid membrane dipole potential by atomic force microscopy. Biophys J 95:5193–5199
Zaharoff DA, Henshaw JW, Mossop B, Yuan F (2008) Mechanistic analysis of electroporation-induced cellular uptake of macromolecules. Exp Biol Med 233:94–105
Acknowledgments
This work was supported by the Excellency Programme of the University of Karlsruhe (Shared Research Group SRG60-1) and a Ph.D. fellowship of the Helmholtz Society to B.F.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flickinger, B., Berghöfer, T., Hohenberger, P. et al. Transmembrane potential measurements on plant cells using the voltage-sensitive dye ANNINE-6. Protoplasma 247, 3–12 (2010). https://doi.org/10.1007/s00709-010-0131-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-010-0131-y