Abstract
Metallic iron (Fe0) is a reactive material that is widely used for industrial water treatment. The course of the metal ion removal process using Fe0 (iron powder) was monitored electrochemically (differential pulse polarography). As probe species, Zn2+, Pb2+, and Cd2+ were selected for their different (1) adsorptive affinity to iron corrosion products (FeCPs), (2) redox properties, (3) precipitation ability at various pH. Batch experiments were carried out with binary (Zn2+/ Pb2+ and Zn2+/ Cd2+) and ternary (Zn2+/Cd2+/Pb2+) systems to reveal the mutual interference of these cations. Detailed time monitoring of iron aging for up to 14 days as well as concentration decay of individual removed cations represent important data for mechanistic discussions. The aqueous concentration of Fe2+ was also monitored. FeCPs were characterized using X-ray photoelectron spectroscopy (XPS) and scanning electron microscopy (SEM). Results showed that the presence of Pb2+ delays the Zn2+ removal whereas the presence of Cd2+ in solution accelerates its removal. The removal of Pb2+ by FeCPs was not affected by the presence of Zn2+ and Cd2+, moreover, the Pb2+ inhibited the effect of Cd2+ on the removal of Zn2+. XPS has proven existence of Fe2O3 and hydrated Fe oxidic phase, whilst the SEM showed that the original Fe grains were partly dissolved into buffered ambient under formation of fine particles of FeCPs. Results confirm that reductive transformation of any contaminant in a Fe0/H2O system is the consequence and not the cause of iron corrosion.
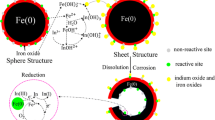
Graphic abstract










Similar content being viewed by others
References
Gosar M (2004) Geoenviron 51:2097
Xiao R, Wang S, Li R, Wang JJ, Zhang Z (2017) Ecotox Environ Safe 141:17
Oldright GL, Keyes HE, Miller V, Sloan WA (1928) Precipitation of lead and copper from solution on sponge iron. Bulletin 281, Bureau of Mines, p 131
Bojic AL, Bojic D, Andjelkovic T (2009) J Hazard Mater 168:813
Vollprecht D, Plessl K, Neuhold S, Kittinger F, Öfner W, Müller P, Mischitz R, Sedlazeck KP (2020) Processes 8:279
Xiao M, Hu R, Cui X, Gwenzi W, Noubactep C (2020) Processes 8:409
O’Hannesin SF, Gillham RW (1998) Ground Water 36:164
Henderson AD, Demond AH (2011) Environ Eng 137:689
Kishimoto N, Iwano S, Narazaki Y (2011) Water Air Soil Pollut 221:183
Guan X, Sun Y, Qin H, Li J, Lo IMC, He D, Dong H (2015) Water Res 75:224
Noubactep C (2015) Water Res 85:114
Suponik T, Winiarski A, Szade J (2015) Water Air Soil Pollut 226:360
Statham TM, Mumford KA, Stark SC, Gore DB, Stevens GW (2015) Sep Sci Technol 50:2427
Vollprecht D, Krois LM, Sedlazeck KP, Müller P, Mischitz R, Olbrich T, Pomberger R (2018) J Clean Prod 208:1409
Devonshire E (1890) J Frankl Inst 129:449
Lauderdale RA, Emmons AH (1951) J Am Water Works Ass 43:327
Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2014) Fresenius Environ Bull 23:2663
Gatcha-Bandjun N, Noubactep C, Loura Mbenguela B (2017) Environ Technol Innov 8:71
Touomo-Wouafo M, Donkeng-Dazie J, Btatkeu-K BD, Tchatchueng JB, Noubactep C, Ludvík J (2018) Chemosphere 209:617
Nde-Tchoupé AI, Nanseu-Njiki CP, Hu R, Nassi A, Noubactep C, Licha T (2019) Chemosphere 219:855
Phukan M, Noubactep C, Licha T (2015) Chem Eng J 259:481
Hildebrant B, Ndé-Tchoupé AI, Lufingo M, Licha T, Noubactep C (2020) Processes 8:265
Noubactep C (2007) Open Environ Sci 1:9
Noubactep C (2008) Environ Technol 29:909
Ghauch A (2015) Freiberg Online Geosci 32:1
Gheju M (2018) Water 10:651
Hu R, Yang H, Tao R, Cui X, Xiao M, Konadu-Amoah B, Cao V, Lufingo M, Soppa-Sangue NP, Ndé-Tchoupé AI, Gatcha-Bandjun N, Sipowo-Tala VR, Gwenzi W, Noubactep C (2020) Water 12:641
Cantrell KJ, Kaplan DI, Wietsma TW (1995) J Hazard Mater 42:201
Qiu SR, Lai HF, Roberson MJ, Hunt ML, Amrhein C, Giancarlo LC, Flynn GW, Yarmoff JA (2000) Langmuir 16:2230
Scott TB, Popescu IC, Crane RA, Noubactep C (2011) J Hazard Mater 186:280
Khorshidi N, Azadmehr AR (2017) Desalin Water Treat 58:106
Christophi CA, Axe L (2000) J Environ Eng 126:66
Forbes EA, Posner AM, Quirk JP (1976) J Soil Sci 27:154
Gadde RR, Laitmen HA (1974) Anal Chem 46:2022
Lavine BK, Auslander G, Ritter J (2001) Microchem J 70:69
Kinraide TB, Yermiyahu U (2007) J Inorg Biochem 101:1201
Nesic S (2007) Corros Sci 49:4308
Lazzari L (2008) General aspects of corrosion. Encyclopedia of hydrocarbons, chapter 9.1, vol V. Istituto Enciclopedia Italiana, Rome
Boparai HK, Joseph M, O’Carroll DM (2013) Environ Sci Pollut Res 20:6210
Pawluk K, Fronczyk J (2015) Pol J Chem Technol 15:7
Wagner CD, Davis LE, Zeller MV, Taylor JA, Raymond RH, Gale LH (1981) Surf Interface Anal 3:211
Xi Y, Mallavarapu M, Naidu R (2010) Mater Res Bull 45:1361
Li XQ, Zhang WX (2007) J Phys Chem C 111:6939
Noubactep C, Btatkeu-K BD, Tchatchueng JB (2011) Chem Eng J 178:78
Kwok RWM (1999) XPSPeak, Version 4.1. Hong Kong, Available online: https://www.phy.cuhk.edu.hk/surface/XPSPeak. Accessed on 21 Sept 2019
Briggs D, Seah MP (1996) Practical surface analysis by Auger and X-ray photoelectron spectroscopy. Wiley, New York
Schultz MF, Benjamin MM, Ferguson JF (1987) Environ Sci Technol 121:863
Benjamin MM (1981) J Coll Inter Sci 79:209
Kinniburgh DG, Jackson ML, Syer JK (1976) Soil Sci Soc Am J 40:796
Acknowledgements
The authors are grateful namely to the institutional support of the J. Heyrovský Institute of Physical Chemistry, Czech Academy of Sciences, RVO 61388955.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Touomo-Wouafo, M., Donkeng-Dazie, J., Jirka, I. et al. Electrochemical monitoring of metal ions removal in Fe0/H2O systems: competitive effects of cations Zn2+, Pb2+, and Cd2+. Monatsh Chem 151, 1511–1523 (2020). https://doi.org/10.1007/s00706-020-02683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02683-6