Abstract
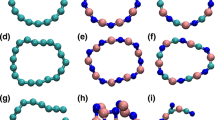
Carbon–nitrogen nanorings with formulae (CN)2n and in the form of [n]pyrazine cyclacenes appear either as closed-shell singlets (SCS) for n = 5, 10, and 12, or open-shell singlets (SOS) for n = 6, 7, 9, and 11 at restricted and unrestricted broken spin-symmetry density functional theory (DFT). All of their corresponding acyclic isomers, which are called [n]pyrazine polyacenes or nanoribbons, appear as SCS for n = 5–12. As a result, nitrogen substitutions alter the electronic ground state of cyclacenes and polyacenes and appear to increase their viability, which invites further experimental and theoretical realization and exploration.
Graphical abstract









Similar content being viewed by others
References
Kawase T, Kurata H (2006) Chem Rev 106:5250
Choi HS, Kim K (1999) Angew Chem Int Ed 38:2256
Kohnke FH, Slawin AMZ, Stoddart JF, Williams DJ (1987) Angew Chem Int Ed Engl 26:892
Ashton PR, Isaacs NS, Kohnke FH, Slawin AMZ, Spencer CM, Stoddart JF, Williams DJ (1988) Angew Chem Int Ed Engl 27:966
Ashton PR, Brown GR, Isaacs NS, Giuffrida D, Kohnke FH, Mathias JP, Slawin AMZ, Smith DR, Stoddart JF, Williams DJ (1992) J Am Chem Soc 114:6330
Cory RM, McPhail CL (1996) Tetrahedron Lett 37:1987
Cory RM, McPhail CL, Dikmans AJ, Vittal JJ (1996) Tetrahedron Lett 37:1983
Godt A, Enkelmann V, Schlüter AD (1989) Angew Chem Int Ed 28:1680
Deichmann M, Nather C, Herges R (2003) Org Lett 5:1269
Scott LT (2003) Angew Chem Int Ed 42:4133 (and references therein)
Vögtle F (1983) Top Curr Chem 115:157
Türker L (1994) Polycyclic Aromatic Compd 4:191
Houk KN, Lee PS, Nendel MJ (2001) Org Chem 66:5517
Loh KP, Yang SW, Soon JM, Zhang H, Wu P (2003) J Phys Chem A 107:5555
Tonmunphean S, Wijitkosoom A, Tantirungrotechai Y, Nuttavut N, Limtrakul J (2003) Bull Chem Soc Jpn 76:1537
Andre JM, Champagne B, Perpete EA, Guillaume M (2001) Int J Quantum Chem 84:607
Türker L, Gümüs S (2004) J Mol Struct (Theochem) 685:1
Kassaee MZ, Arefrad H, Ghambarian M (2008) Int J Quantum Chem 108:696
Chen Z, Jiang D, Lu X, Bettinger HF, Dai S, Schleyer PvR, Houk KN (2007) Org Lett 9:5449
Sadowsky D, McNeill K, Cramer CJ (2010) Faraday Discuss 145:1
Esser B, Rskatov JA, Gleiter R (2007) Org Lett 9:4037
Esser B, Rominger F, Gleiter R (2008) J Am Chem Soc 130:6716
Kornmayer SC, Esser B, Gleiter R (2009) Org Lett 11:725
Jiang DE, Dai S (2008) J Phys Chem A 112:332
Clar E (1964) Polycyclic hydrocarbons, vols 1, 2. Academic, London
Geerts Y, Klärner G, Müllen K (1998) In: Müllen K, Wagner G (eds) Electronic materials: the oligomer approach. Wiley, Weinheim, p 48
Dimitrakopoulos CD, Malenfant PRL (2002) Adv Mater 14:99
Hegmann FA, Tykwinski RR, Lui KPH, Bullock JE, Anthony JE (2002) Phys Rev Lett 89:227403–1/4
Meng H, Bendikov M, Mitchell G, Helgeson R, Wudl F, Bao Z, Siegrist T, Kloc C, Chen C-H (2003) Adv Mater 15:1090
Payne MM, Parkin SR, Anthony JE (2005) J Am Chem Soc 127:8028
Chun D, Cheng Y, Wudl F (2008) Angew Chem Int Ed 47:8380
Kaur I, Stein NN, Kopreski RP, Miller GP (2009) J Am Chem Soc 131:3424
Kertesz M, Hoffmann R (1983) Solid State Commun 47:97
Lowe JP, Kafafi SA, LaFemina JP (1986) J Phys Chem 90:6602
Kivelson S, Chapman OL (1983) Phys Rev B 28:7236
Wiberg K (1997) J Org Chem 62:5720
Schleyer PvR, Manoharan M, Jiao H, Stahl F (2001) Org Lett 3:3643
Raghu C, Pati YA, Ramasesha S (2002) Phys Rev B 65:155204/1–9
Angliker H, Rommel E, Wirz J (1982) Chem Phys Lett 87:208
McMaster DR, Wirz J (2001) J Am Chem Soc 123:238
Benikov M, Duong HM, Starkey K, Houk KN, Carter EA, Wudl F (2004) J Am Chem Soc 126:7416
Hachman J, Dorando JJ, Avilés M, Chan GKL (2007) J Chem Phys 127:134309
Qu Z, Zhang D, Liu C, Jiang Y (2009) J Phys Chem A 113:7909
Fujita M, Wakabayashi K, Nakada K, Kusakabe K (1996) J Phys Soc Jpn 65:1920
Enoki T, Kobayashi Y (2005) J Mater Chem 15:3999
Ezawa M (2006) Phys Rev B 73:045432
Son Y-W, Cohen ML, Louie SG (2006) Nature 444:347
Kim WY, Kim KS (2008) J Comput Chem 29:1073
Kim WY, Kim KS (2008) Nature Nanotech 3:408
Kim WY, Choi YC, Kim KS (2008) J Mater Chem 18:4510
Jiang DE, Sumpter BG, Dai S (2007) J Chem Phys 126:134701
Türker L, Gümüs S (2004) J Mol Struct 679:143 (Theochem)
Winkler M, Houk KN (2007) J Am Chem Soc 129:1805
Tonzola CJ, Alam MM, Kaminsky W, Jenekhe SA (2003) J Am Chem Soc 125:13548
Nishida J-I, Naraso MS, Fujiwara E, Tada H, Tomura M, Yamashita Y (2004) Org Lett 6:2007
Miao S, Appleton AL, Berger N, Barlow S, Marder SR, Hardcastle KI, Bunz UHF (2009) Chem Eur J 15:4990
Tang Q, Liu J, Chan HS, Miao Q (2009) Chem Eur J 15:3965
Yu SS, Zheng WT, Wen QB, Jiang Q (2008) Carbon 46:537
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford
Koch W, Holthausen MC (2000) A chemists guide to density functional theory. Wiley, Weinheim
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B Condens Matter 37:785
Krishnan K, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG Jr, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Rev. A 7. Gaussian, Pittsburgh
Carpenter JE, Weinhold F (1988) J Mol Struct (Theochem) 41:169
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2010) NBO version 3.1
Schleyer PvR, Maerker C, Dransfeld A, Jiao H, NJRvE Hommes (1996) J Am Chem Soc 118:6317
Schleyer PvR, Jiao H, Hommes NJRvE, Malkin VG, Malkina OL (1997) J Am Chem Soc 119:12669
Jiang DE, Sumpter BG, Dai S (2007) J Chem Phys 127:124703
Du P, Hrovat DA, Borden WT, Lahti PM, Rossi AR, Berson JA (1986) J Am Chem Soc 108:5072
Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PvR (2005) Chem Rev 105:3842
Zhou Z, Zhao J, Gao X, Chen Z, Yan J, Schleyer PvR, Morinaga M (2005) Chem Mater 17:992
Mpourmpakis G, Froudakis GE, Lithoxoos GP, Samios J (2006) Nano Lett 6:1581
Acknowledgment
We thank Mr. M. Ghambarian from Tarbiat Modares University for his stimulating and helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material, which contains Cartesian coordinates, absolute energies, energy differences between different electronic states, bond lengths, spin contaminations, molecular orbital diagrams, frontier molecular orbital of [6]pyrazine cyclacene and [5]pyrazine polyacene, NICS values of pyrazine nanorings for all calculated compounds at the B3LYP/6-31G* level.
Rights and permissions
About this article
Cite this article
Kassaee, M.Z., Aref Rad, H. & Soleimani Amiri, S. Carbon–nitrogen nanorings and nanoribbons: a theoretical approach for altering the ground states of cyclacenes and polyacenes. Monatsh Chem 141, 1313–1319 (2010). https://doi.org/10.1007/s00706-010-0398-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0398-x