Abstract
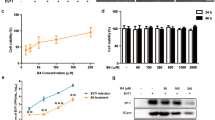
Enterovirus 71 (EV71) poses a major threat to public health globally due to severe and even fatal hand, foot, and mouth disease (HFMD). However, no effective antiviral agents are available to treat HFMD caused by this virus. Polysaccharides have been shown to exhibit antiviral activity, and polysaccharides extracted from Picochlorum sp. 122 (PPE) could potentially be used to treat HFMD, but reports on their antiviral activity are limited. In this study, the antiviral activity of PPE against EV71 was verified in Vero cells. PPE was shown to limit EV71 infection, as demonstrated using an MTT assay and by observing the cellular cytopathic effect. In addition, a decrease in VP1 RNA and protein levels indicated that PPE effectively inhibits proliferation of EV71 in Vero cells. An annexin V affinity assay also indicated that PPE protects host cells from apoptosis through the AKT and ATM/ATR signalling pathways. These results demonstrate that PPE has potential as an antiviral drug to treat HFMD caused by EV71.






Similar content being viewed by others
References
Xu Z, Hu W, Jiao K, Ren C, Jiang B, Ma W (2019) The effect of temperature on childhood hand, foot and mouth disease in Guangdong Province, China, 2010–2013: a multicity study. BMC Infect Dis 19:969–969
Gonzalez G, Carr MJ, Kobayashi M, Hanaoka N, Fujimoto T (2019) Enterovirus-associated hand-foot and mouth disease and neurological complications in japan and the rest of the World. Int J Mol Sci 20:1–6
Wang Y, Zhao H, Ou R, Zhu H, Gan L, Zeng Z, Yuan R, Yu H, Ye M (2020) Epidemiological and clinical characteristics of severe hand-foot-and-mouth disease (HFMD) among children: a 6-year population-based study. BMC Public Health 20:801–801
Chen L, Xu SJ, Yao XJ, Yang H, Zhang HL, Meng J, Zeng HR, Huang XH, Zhang RL, He YQ (2020) Molecular epidemiology of enteroviruses associated with severe hand, foot and mouth disease in Shenzhen, China, 2014–2018. Arch Virol 165:2213–2227
Phanthong S, Densumite J, Seesuay W, Thanongsaksrikul J, Teimoori S, Sookrung N, Poovorawan Y, Onvimala N, Guntapong R, Pattanapanyasat K, Chaicumpa W (2020) Human antibodies to VP4 inhibit replication of enteroviruses across subgenotypes and serotypes, and enhance host innate immunity. Front Microbiol 11:562768
Schmidt NJ, Lennette EH, Ho HH (1974) An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 129:304–309
Sun H, Gao M, Cui D (2020) Molecular characteristics of the VP1 region of enterovirus 71 strains in China. Gut Pathog 12:1–11
Solomon T, Lewthwaite P, Perera D, Cardosa MJ, Mcminn P, Ooi MH (2010) Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 10:778–790
Wang MG, Sun HM, Liu XM, Deng XQ (2017) Clinical analysis of 59 children with hand foot and mouth diseases due to enterovirus EV71 and concomitant viral encephalitis. Eur Rev Med Pharmacol Sci 21:43–49
Yang Z, Gao F, Wang X, Shi L, Zhou Z, Jiang Y, Ma X, Zhang C, Zhou C, Zeng X, Liu G, Fan J, Mao Q, Shi L (2020) Development and characterization of an enterovirus 71 (EV71) virus-like particles (VLPs) vaccine produced in Pichia pastoris. Hum Vaccin Immunother 16:1602–1610
Chan TC, Hwang JS, Chen RH, King CC, Chiang PH (2014) Spatio-temporal analysis on enterovirus cases through integrated surveillance in Taiwan. BMC Public Health 14:11–11
Lum LC, Wong KT, Lam SK, Chua KB, Goh AY, Lim WL, Ong BB, Paul G, Abubakar S, Lambert M (1998) Fatal enterovirus 71 encephalomyelitis. J Pediatr 133:795–798
Takechi M, Fukushima W, Nakano T, Inui M, Ohfuji S, Kase T, Ito K, Kondo K, Maeda A, Shimizu H, Hirota Y (2019) Nationwide survey of pediatric inpatients with hand, foot, and mouth disease, herpangina, and associated complications during an epidemic period in Japan: Estimated number of hospitalized patients and factors associated with severe cases. J Epidemiol 29:354–362
Singh S, Chow VT, Phoon MC, Chan KP, Poh CL (2002) Direct detection of enterovirus 71 (EV71) in clinical specimens from a hand, foot, and mouth disease outbreak in Singapore by reverse transcription-PCR with universal enterovirus and EV71-specific primers. J Clin Microbiol 40:2823–2827
Nguyen NT, Pham HV, Hoang CQ, Nguyen TM, Nguyen LT, Phan HC, Phan LT, Vu LN, Tran MN (2014) Epidemiological and clinical characteristics of children who died from hand, foot and mouth disease in Vietnam, 2011. Bmc Infect Dis 14:341–341
Horwood PF, Andronico A, Tarantola A, Salje H, Duong V, Mey C, Ly S, Dussart P, Cauchemez S, Buchy P (2016) Seroepidemiology of human enterovirus 71 infection among children, Cambodia. Emerg Infect Dis 22:92–95
Wijffels RH, Barbosa MJ (2010) An outlook on microalgal biofuels. Science 329:796–799
Lian J, Wijffels RH, Smidt H, Sipkema D (2018) The effect of the algal microbiome on industrial production of microalgae. Microb Biotechnol 11:806–818
de Jesus RM, de Morais AM, de Morais RM (2015) Marine polysaccharides from algae with potential biomedical applications. Mar Drugs 13:2967–3028
Kholiya F, Chatterjee S, Bhojani G, Sen S, Barkume M, Kasinathan NK, Kode J, Meena R (2020) Seaweed polysaccharide derived bioaldehyde nanocomposite: Potential application in anticancer therapeutics. Carbohydr Polym 240:116282
Okimura T, Jiang Z, Komatsubara H, Hirasaka K, Oda T (2020) Therapeutic effects of an orally administered edible seaweed-derived polysaccharide preparation, ascophyllan HS, on a Streptococcus pneumoniae infection mouse model. Int J Biol Macromol 154:1116–1122
Abu-Galiyun E, Huleihel M, Levy-Ontman O (2019) Antiviral bioactivity of renewable polysaccharides against Varicella Zoster. Cell Cycle 18:3540–3549
Li H, Bai Z, Li C, Sheng C, Zhao X (2020) EV71 infection induces cell apoptosis through ROS generation and SIRT1 activation. J Cell Biochem 121:4321–4331
You L, Chen J, Liu W, Xiang Q, Luo Z, Wang W, Xu W, Wu K, Zhang Q, Liu Y, Wu J (2020) Enterovirus 71 induces neural cell apoptosis and autophagy through promoting ACOX1 downregulation and ROS generation. Virulence 11:537–553
Du X, Wang H, Xu F, Huang Y, Liu Z, Liu T (2015) Enterovirus 71 induces apoptosis of SHSY5Y human neuroblastoma cells through stimulation of endogenous microRNA let-7b expression. Mol Med Rep 12:953–959
Molloy SD, Pietrak MR, Bricknell I, Bouchard DA (2013) Experimental transmission of infectious pancreatic necrosis virus from the blue mussel, Mytilus edulis, to cohabitating Atlantic Salmon (Salmo salar) smolts. Appl Environ Microbiol 79:5882–5890
Wang C, Chen H, Chen D, Zhao M, Lin Z, Guo M, Xu T, Chen Y, Hua L, Lin T, Tang Y, Zhu B, Li Y (2020) The inhibition of H1N1 influenza virus-induced apoptosis by surface decoration of selenium nanoparticles with beta-thujaplicin through reactive oxygen species-mediated AKT and p53 signaling pathways. ACS Omega 5:30633–30642
Zhong J, Xia Y, Hua L, Liu X, Xiao M, Xu T, Zhu B, Cao H (2019) Functionalized selenium nanoparticles enhance the anti-EV71 activity of oseltamivir in human astrocytoma cell model. Artif Cells Nanomed Biotechnol 47:3485–3491
Guo M, Li Y, Lin Z, Zhao M, Xiao M, Wang C, Xu T, Xia Y, Zhu B (2017) Surface decoration of selenium nanoparticles with curcumin induced HepG2 cell apoptosis through ROS mediated p53 and AKT signaling pathways. Rsc Adv 7:52456–52464
Li Y, Guo M, Lin Z, Zhao M, Xiao M, Wang C, Xu T, Chen T, Zhu B (2016) Polyethylenimine-functionalized silver nanoparticle-based co-delivery of paclitaxel to induce HepG2 cell apoptosis. Int J Nanomedicine 11:6693–6702
Plevka P, Perera R, Cardosa J, Kuhn RJ, Rossmann MG (2012) Crystal structure of human enterovirus 71. Science 336:1274–1274
Meng T, Jia Q, Wong SM, Chua KB (2019) In vitro and in vivo inhibition of the infectivity of human enterovirus 71 by a sulfonated food azo dye, Brilliant Black BN. J Virol 93:e00061-e19
Wang R, Weng KF, Huang YC, Chen CJ (2016) Elevated expression of circulating miR876-5p is a specific response to severe EV71 infections. Sci Rep 6:24149
Acknowledgements
This work was supported by the Pediatrics Institute Foundation of Guangzhou Women and Children’s Medical Centre (YIP-2019-059), the Guangdong Natural Science Foundation (2020A1515110648), the Open Fund of Guangdong Provincial Key Laboratory of Functional Supramolecular Coordination Materials and Applications (2020A03), the Open Project of Guangdong Key Laboratory of Marine Materia Medica (LMM2020-7), the Technology Planning Project of Guangzhou (202102010202), and the Guangzhou Medical University Students’ Science and Technology Innovation Project (2019AEK02, 2020AEK03, 2020AEK06, 2020AEK12, 2021AEK119, 2021AEK122, 2021AEK125 and 2021AEK128).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest in this work.
Additional information
Handling Editor: Akbar Dastjerdi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, M., Zheng, R., Wu, Hl. et al. Inhibition of enterovirus 71 infection by polysaccharides extracted from Picochlorum sp. 122 via the AKT and ATM/ATR signaling pathways. Arch Virol 166, 3269–3274 (2021). https://doi.org/10.1007/s00705-021-05229-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-021-05229-1