Abstract
Barley stripe mosaic virus (BSMV) is an important seed-transmitted pathogen occurring worldwide. Recently, the occurrence of mild BSMV pathotypes has been observed in barley crops in Poland. In this study, the full-length genome sequences of mild and aggressive Polish and German BSMV isolates was established. Phylogenetic and recombination analysis was performed using Polish and other BSMV isolates described to date. The analysis revealed that Polish isolates differed only in 25 nucleotides, which suggests that point mutations might have had a great impact on the biological properties of the virus. The phylogenetic analysis revealed that the closest relationship was that between European and BSMV-CV42, BSMV-ND18 and BSMV-Type isolates, whereas the highest genetic distance was observed for BSMV-Qasr Ibrim and BSMV-China isolates. A recombination event within the αa protein of BSMV-De-M and BSMV-CV42 isolates was also detected. Moreover, a sensitive reverse transcription loop-mediated isothermal amplification (RT-LAMP) method was developed for rapid detection of BSMV isolates. The RT-LAMP assay can be used for routine diagnostics of BSMV in seed and plant material.



Similar content being viewed by others
References
Adams MJ, Heinze C, Jackson AO, Kreuze JF, MacFarlane SA, Torrance L (2012) Family Virgaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz E (eds) Virus taxonomy: ninth report of the international committee on taxonomy of viruses. Elsevier, London, pp 1139–1162
Jackson AO, Lim H-S, Bragg JN, Ganesan U, Lee MY (2009) Hordeivirus replication, movement and pathogenesis. Annu Rev Phytopathol 47:385–422
Carroll TW (1980) Barley stripe mosaic virus: its economic importance and control. Plant Dis 64:136–140
Chiko AW, Baker RJ (1978) Economic significance of Barley stripe mosaic virus in Canadian prairies. Can J Plant Sci 58:331–340
Carroll TW (1983) Certification schemes against barley stripe mosaic. Seed Sci Tech 11:1033–1042
OEPP, EPPO (1983) Data sheets on quarantine organisms. No. 88, Barley stripe mosaic virus. Bull OEPP 13:1–5
Jackson AO, Petty ITD, Jones RW, Edwards MC, French R (1991) Analysis of Barley stripe mosaic virus pathogenicity. Sem Virol 2:107–119
Petty IT, Hunter BG, Jackson AO (1988) A novel strategy for one-step cloning of full-length cDNA and its application to the genome of Barley stripe mosaic virus. Gene 74:423–432
Weiland JJ, Edwards MC (1994) Evidence that the αa gene of Barley stripe mosaic virus encodes determinants of pathogenicity to oat (Avena sativa). Virology 201:116–126
Edwards MC (1995) Mapping of the seed transmission determinants of Barley stripe mosaic virus. Mol Plant Microbe Interact 8:906–915
Smith O, Clapham A, Rose P, Liu Y, Wang J, Allaby RG (2014) A complete ancient RNA genome: identification, reconstruction and evolutionary history of archeological Barley stripe mosaic virus. Sci Rep 4:4003
Herrera GM, Beratto EM, Andrade OV, Madariaga MV (2001) Identification of Barley stripe mosaic virus (BSMV) on barley in Chile. Agri Técn 61:275–280
Köklü G (2004) Occurrence of cereal viruses on wheat in Tekirdag, Turkey. Phytoprotection 85:19–25
Kapooria RG, Ndunguru J (2004) Occurrence of viruses in irrigated wheat in Zambia. Bull OEPP 34:413–419
Hafez EE, Engy E, Aleem A, Fattouh FA (2008) Comparison of Barley stripe mosaic virus strains. Z Nat 63:271–276
Lim H-J, Seo A-Y, Kim C-S, Kim J-K, Park C-H, Gong J-S, Kim I-H, Han S-H, Kilcrease JP, Furuya N, Tsuchiya K, Hammond J, Lim H-S (2016) Nationwide survey revealed Barley stripe mosaic virus in Korean barley fields. J Fac Agr Kyushu U 61:71–77
Jeżewska M (2001) Identification of Barley stripe mosaic virus in Poland. J Plant Prot Res 41:164–167
Jeżewska M (2006) Seed-transmitted cereal viruses—occurrence in Poland and their potential harmfulness. Dissertation. Institute of Plant Protection–National Research Institute
Zarzyńska A, Jeżewska M, Trzmiel K, Hasiów-Jaroszewska B (2014) Development of a one step immunocapture real-time RT-PCR assay for the detection of Barley stripe mosaic virus strains in barley seedlings. Acta Virol 58:81–85
Zarzyńska-Nowak A, Jeżewska M, Hasiów-Jaroszewska B, Zielińska L (2015) A comparison of ultrastructural changes of barley cells infected with mild and aggressive isolates of Barley stripe mosaic virus. J Plant Dis Prot 122:153–160
Zhao K, Liu Y, Wang X (2010) Reverse transcription loop-mediated isothermal amplification of DNA for detection of Barley yellow dwarf viruses in China. J Virol Methods 169:211–214
Le DT, Netsu O, Uehara-Ichiki T, Shimizu T, Choi I-R, Omura T, Sasaya T (2010) Molecular detection of nine rice viruses by a reverse-transcription loop-mediated isothermal amplification assay. J Virol Methods 170:90–93
Fukuta S, Tamura M, Maejima H, Takahashi R, Kuwayama S, Tsuji T, Yoshida T, Itoh K, Hashizume H, Nakajima Y, Uehara Y, Shirako Y (2013) Differential detection of Wheat yellow mosaic virus, Japanese soil-borne wheat mosaic virus and Chinese wheat mosaic virus by reverse transcription loop-mediated isothermal amplification reaction. J Virol Methods 189:348–354
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acid Symp 41:95–98
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:609–612
Delport W, Poon AFY, Frost SDW, Pond SLK (2010) Datamonkey 2010: a suite of phylogenetic analysis tools for evolutionary biology. Bioinformatics 26:2455–2457
Martin DP, Murrell B, Golden M, Khoosal A, Muhire B (2015) RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol 1:1–5
Huson DH (1998) SplitsTree: a program for analyzing and visualizing evolutionary data. Bioinformatics 14:68–73
McFarland JE, Brakke MK, Jackson AO (1983) Complexity of the Argentina mild strain of Barley stripe mosaic virus. Virology 130:397–402
Zhou H, Jackson AO (1996) Analysis of cis-acting elements required for replication of Barley stripe mosaic virus RNAs. Virology 219:150–160
Johnson AJ, Bragg JN, Lawrence DM, Jackson AO (2003) Sequence elements controlling expression of Barley stripe mosaic virus subgenomic RNAs in vivo. Virology 313:66–80
Donald RGK, Jackson AO (1994) The Barley stripe mosaic virus γb gene encodes a multifunctional cysteine-rich protein that affects pathogenesis. Plant Cell 6:1593–1606
Petty ITD, Hunter BG, Wei N, Jackson AO (1989) Infectious Barley stripe mosaic virus RNA transcribed from full-length genomic cDNA clones. Virology 171:342–349
Petty ITD, Donald RGK, Jackson AO (1994) Multiple genetic determinants of Barley stripe mosaic virus influence lesion phenotype on Chenopodium amaranticolor. Virology 198:218–226
Weiland JJ, Edwards MC (1996) A single nucleotide substitution in the αa gene confers oat pathogenicity to Barley stripe mosaic virus strain ND18. Mol Plant Microbe Interact 9:62–67
Yang Z, Nielsen R, Goldman N, Pedersen A-MK (2000) Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155:431–449
Vigne E, Marmonier A, Fuchs A (2008) Multiple interspecies recombination events within RNA2 of Grapevine fanleaf virus and Arabis mosaic virus. Arch Virol 153:1771–1776
Sanjuán R, Domingo-Calap P (2016) Mechanisms of viral mutation. Cell Mol Life Sci 73:4433–4448
Edwards MC, Petty ITD, Jackson AO (1992) RNA recombination in the genome of Barley stripe mosaic virus. Virology 189:389–392
Acknowledgements
We would like to thank Dr. Frank Rabenstein for providing the German isolate of BSMV used in this study. This study is part of a project supported by the Polish National Science Centre [2015/16/T/NZ9/00406].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by F. Murilo Zerbini.
Electronic supplementary material
Below is the link to the electronic supplementary material.
705_2018_3725_MOESM2_ESM.tif
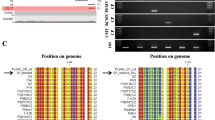
Supplementary Figure: Detection of BSMV isolates by RT-LAMP. (a) Visual detection of amplification products under UV light. A positive reaction is indicated by a fluorescent bright green color, while the negative control remains orange. (b) Electrophoretic separation of RT-LAMP products. M-Kb Plus DNA Ladder (ThermoFisher Scientific), lines 1-5: BSMV-R samples, lines 6-10: BSMV-M, lines 11-15: BSMV-De-M, NC-negative control (healthy plant) (TIFF 2430 kb)
Rights and permissions
About this article
Cite this article
Zarzyńska-Nowak, A., Hasiów-Jaroszewska, B. & Jeżewska, M. Molecular analysis of barley stripe mosaic virus isolates differing in their biological properties and the development of reverse transcription loop-mediated isothermal amplification assays for their detection. Arch Virol 163, 1163–1170 (2018). https://doi.org/10.1007/s00705-018-3725-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-3725-x