Abstract
The discovery of glucocerebrosidase (GBA1) mutations as the greatest numerical genetic risk factor for the development of Parkinson disease (PD) resulted in a paradigm shift within the research landscape. Efforts to elucidate the mechanisms behind GBA1-associated PD have highlighted shared pathways in idiopathic PD including the loss and gain-of-function hypotheses, endoplasmic reticulum stress, lipid metabolism, neuroinflammation, mitochondrial dysfunction and altered autophagy–lysosomal pathway responsible for degradation of aggregated and misfolded a-synuclein. GBA1-associated PD exhibits subtle differences in phenotype and disease progression compared to idiopathic counterparts notably an earlier age of onset, faster motor decline and greater frequency of non-motor symptoms (which also constitute a significant aspect of the prodromal phase of the disease). GBA1-targeted therapies have been developed and are being investigated in clinical trials. The most notable are Ambroxol, a small molecule chaperone, and Venglustat, a blood–brain-barrier-penetrant substrate reduction therapy agent. It is imperative that further studies clarify the aetiology of GBA1-associated PD, enabling the development of a greater abundance of targeted therapies in this new era of precision medicine.
Similar content being viewed by others
Introduction
Biallelic (homozygous or compound heterozygous) mutations in the GBA1 gene, encoding the lysosomal enzyme glucocerebrosidase (GCase; EC 3.2.1.45), are pathognomonic for the commonest lysosomal storage disorder (LSD) Gaucher disease (GD) (Grabowski 2008). Heterozygous GBA1 variants account for the most significant genetic risk factor for Parkinson disease (PD), the second most common neurodegenerative disease following Alzheimer’s disease with a reported prevalence rate of 315 per 100,000 persons worldwide (Sidransky et al. 2009; Pringsheim et al. 2014). Approximately, 5–30% of PD patients carry GBA1 mutations; variations in the prevalence rate of GBA1 mutations can be attributed to the population studied and extent of exome sequencing (Sidransky et al. 2009; Duran et al. 2013; Schapira 2015; Cilia et al. 2016, Senkevich and Gan-Or 2020). Clinically, GBA1-associated PD (GBA1-PD) mirrors idiopathic PD (iPD), albeit significant differences include an earlier age of disease onset, greater frequency of non-motor symptoms (NMSs) and cognitive impairment (Petrucci et al. 2020; Avenali et al. 2021). GBA1 mutation-positive individuals exhibit an increased risk of developing Dementia with Lewy Bodies (DLB; odds ratio, OR ~ 8), notably higher than that for PD (OR 3.5–6) (Neumann et al. 2009; Sidransky et al. 2009; McKeith et al. 2017). In this review, we provide an update on the association between GBA1 mutations and PD and how future neuroprotective therapies may target the GBA1 pathway.
GBA1 mutations and Gaucher disease
Biallelic GBA1 mutations cause GD, the commonest LSD, characterised by GCase deficiency. The enzyme GCase is involved in glycosphingolipid and ganglioside metabolism, cleaving glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph) into ceramide and glucose, and sphingosine and glucose, respectively (Brady et al. 1965). Reduced GCase activity leads to GlcCer and GlcSph accumulation in the Gaucher cells (lysosomes of macrophages) within the liver, bone marrow and spleen (Rosenbloom and Weinreb 2013). GD is particularly prevalent in the Ashkenazi Jewish (AJ) population (118 per 100,000) compared to non-AJ populations (1–2 per 100,000) (Mistry et al. 2011). GD presents with variable symptomatology; the highly heterogeneous disease can be stratified into three types according to severity and neurological involvement. Type I (non-neuronopathic) GD accounts for 95% of cases with a spectrum of clinical presentations from subclinical patients to hallmark symptoms of hepatosplenomegaly, pancytopenia and osteoporosis (Grabowski et al. 2015). Neuronopathic GD is categorised into patient subgroups demonstrating rapid (type 2 GD) and slower (type 3 GD) neurological deterioration (Mistry et al. 2015). Neurological symptoms include myoclonic or generalised seizures and eye movement abnormalities (saccades and supranuclear ophthalmoplegia) (Mistry et al. 2015). Different GBA1 mutations are more represented in specific phenotypes of GD. The ‘mild’ GD type 1 is found with the N370S GBA1 mutation and a proportion of homozygous N370S GBA1 mutation-positive patients remain asymptomatic (Hruska et al. 2008). The L444P GBA1 mutation has been reported in patients exhibiting severe neurological symptoms, accounting for over 40% of mutations in neuronopathic forms of GD (Koprivica et al. 2000; Stone et al. 2000). Such ‘mild’ and ‘severe’ GBA1 mutations are associated with an in vitro residual GCase enzymatic activity of 32–38% and 13–24%, respectively (Alfonso et al. 2004; Sidransky and Lopez 2012; Malini et al. 2014). Others have failed to replicate such findings, instead reporting an overlap in the range of GCase enzymatic activity for severe and mild GBA1 mutations (Sidransky 2004). Analyses of GCase activity within the lysosome only may offer more accurate and consistent readings. The wide spectrum of clinical manifestations of GD hinders a clear-cut classification of GD in practice, particularly for neuronopathic forms of GD (Goker-Alpan et al. 2003). Mounting evidence supports the notion that GD is likely to be a spectrum disorder instead (Beavan et al. 2015). Current treatments for GD aim to enhance GCase activity via enzyme replacement therapy (ERT) and substrate reduction therapy (SRT). Newer techniques, such as, chaperone molecules are under current investigation (Parenti 2009). Administered intravenously, ERT supplies recombinant GCase enzymes, modified to facilitate efficient uptake via macrophages. ERT is a treatment option in type 1 GD, but since ERT cannot penetrate the blood–brain barrier (BBB), its potential use in ameliorating neurological involvement in GD is limited (Valayannopoulos 2013; Jung et al. 2016). A more upstream therapy, SRT, utilises GlcCer synthase inhibitors to reduce GlcCer accumulation (Lukina et al. 2019).
The GBA1 gene localises to chromosome 1q21, comprising 7 kb with 11 exons and 10 introns. A highly homologous pseudogene, GBA1P (with 96% exonic sequence homology), is located 12 kb downstream, enabling recombination events between GBA1P and GBA1 which generate multiple complex alleles (Hruska et al. 2008). Over 300 GBA1 mutations have been reported; more than 100 GBA1 variants, not termed mutations as they are not pathogenic for GD, are associated with PD (Guo et al. 2015; Fan et al. 2016; O'Regan et al. 2017). 90% of GBA1 mutations in AJ patients are N370S (or N409S according to the latest nomenclature), L444P (L483P), IVS2 + 1G > A or 84insG (Gomez et al. 2017). Interestingly, GBA1 mutations exhibit ethnic heterogeneity (Zhang et al. 2018a). N370S and L444P GBA1 mutations are frequently detected in AJ GD patients and non-AJ Caucasians, respectively (Grabowski and Horowitz 1997; Hruska et al. 2008). Asian ethnic groups frequently harbour L444P and F252I GBA1 mutations in neuronopathic GD subtypes, with N370S rarely found (Hruska et al. 2008; Riboldi and Di Fonzo 2019). Over the last two decades, an association between GD and PD has been noticed, initially through case reports of parkinsonian symptoms among GD patients (Tayebi et al. 2003; Aharon-Peretz et al. 2004; Beavan et al. 2015) and confirmed by a large multicentre study (Sidransky et al. 2009).
The mature GCase protein is a 497-amino-acid protein assembled on endoplasmic reticulum (ER) and trafficked to the lysosome by binding to the LIMP2 receptor where it is activated by its cofactor, Saposin C (Grabowski et al. 1990). Three structural domains of GCase have been identified using X-crystallography: domain I contains an antiparallel beta-sheet and structural loop (residues 1–27 and 383–414); domain II made up of eight beta-sheets, resembling an immunoglobulin fold (residues 30–75 and 431–497) and domain III harbours the active site within a (β/α)8 triophosphate isomerase (TIM) barrel (residues 76–381 and 416–430) (Kacher et al. 2008).
GBA1 mutations and Parkinson disease
PD is a common neurodegenerative disorder characterised by a progressive loss of dopaminergic neurones in the substantia nigra pars compacta (SNpc) and intracytoplasmic inclusions predominantly composed of aggregated a-synuclein (Lewy bodies) (Pellicano et al. 2007). PD is clinically defined by the presence of the cardinal motor symptoms of rigidity, bradykinesia and tremor (Postuma et al. 2015). NMSs, such as neuropsychiatric symptoms, autonomic dysfunction, hyposmia, rapid eye movement sleep disorder (RBD) and cognitive impairment, are a prominent feature of PD but remain largely resistant to dopaminergic therapy given the involvement of other neurotransmitters (Schapira et al. 2017). Recent studies have highlighted a prodromal phase, characterised by NMSs and mild motor signs, which may precede clinical diagnosis of PD by up to 20 years (Gustafsson et al. 2015; Schrag et al. 2015; Fereshtehnejad et al. 2019). Accurate identification of the prodromal phase will be critical for the efficacy of potential neuroprotective therapies given that 50–70% of SNpc dopaminergic neurones have already undergone degeneration prior to the onset of motor symptoms (Dauer and Przedborski 2003; Ross et al. 2004).
The association between GBA1 mutations and PD was first observed in clinic over 20 years ago following reports of parkinsonian features in GD patients and GBA1 mutation carriers (Neudorfer et al. 1996; Machaczka et al. 1999). Tayebi et al. (2003) revealed the presence of non-neuronopathic N370S GBA1 mutations in GD patients with Parkinsonism. Autopsy studies of GD brains added further support to the GD–PD association through findings of a significant loss of substantia nigra dopaminergic neurones alongside extensive cytotoxic Lewy body aggregation (Tayebi et al. 2003; Wong et al. 2004). Confirmation of this genetic link was later provided by a large multicentre study of 5691 PD patients and 4898 controls, with an OR of 5.43 for GBA1 (Sidransky et al. 2009). Subsequent genetic analyses replicated such results, demonstrating that GBA1 mutations represent the greatest numerical genetic risk factor for PD (Chen et al. 2014; Nalls et al. 2014; Robak et al. 2017). Both homozygous and heterozygous GBA1 mutations have similar ORs for PD (Alcalay et al. 2014). Penetrance of GBA1 mutations is variable and age dependent; a cumulative risk of developing PD of 5% and 10–30% is observed by 60 and 80 years of age, respectively, in heterozygous carriers when compared to controls (Anheim et al. 2012; Rana et al. 2013; Balestrino et al. 2020). Causes underlying the relatively low rate of PD phenoconversion in individuals with biallelic or heterozygous GBA1 mutations remain elusive (Anheim et al. 2012). Ethnicity also influences the extent by which GBA1 mutations result in an increased PD risk (Zhang et al. 2018a). PD risk is associated with the following GBA1 mutations: R496H and 84insGG in AJ populations; L444P, R120W, IVS2 + 1G > A, H255Q, D409H, RecNciI, E326K, T369M in non-AJ populations; N370S, H255Q, D409H, E326K in European/West Asians; R120W in East Asians, while N370S conveys a pan-ethnic PD risk (Zhang et al. 2018a).
Features of GBA1-associated Parkinson disease
Clinical features
Initial studies reported no significant differences in clinical trajectories between iPD and GBA1-PD (Aharon-Peretz et al. 2005). Key subtle traits of GBA1-PD have since emerged notably, an earlier age of onset by ~ 1.7–6.0 years, higher Unified Parkinson’s Disease Rating Scale Part III (UPDRS-III) scores, greater frequency of dementia, visual hallucinations and severity of NMSs, particularly depression (Neumann et al. 2009; Hu et al. 2010; Winder-Rhodes et al. 2013; Asselta et al. 2014; Zhang et al. 2018b). Notably, there is no apparent correlation between PD clinical phenotype or severity and GCase activity (Alcalay et al. 2020; Omer et al. 2022), although cognitive dysfunction may be an exception to this (see below).
Clinically, GBA1-PD patients exhibit motor symptoms of tremor, rigidity, and bradykinesia, with the latter more commonly observed in the earliest phases of disease in this cohort (Ziegler et al. 2007; Lesage et al. 2011). A faster progression and deterioration in disease course in GBA1-PD patients has been reported extensively in literature, concomitant with a reduced survival rate, compared to iPD (Winder-Rhodes et al. 2013; Brockmann et al. 2015). Interestingly, non-pathogenic GBA1 variants may indeed affect motor symptomatology, with polymorphisms also associated with a greater risk of motor deterioration (Winder-Rhodes et al. 2013; Jesus et al. 2016). Motor complications, wearing off, delayed on and dyskinesias, are more prevalent in GBA1-PD, especially in those harbouring severe mutations, and occur earlier than in GBA1 mutation-negative PD patients (Jesus et al. 2016; Zhang et al. 2018b).
NMSs form a significant aspect of the GBA1-PD disease course. PD patients with GBA1 mutations exhibit a threefold increased risk of cognitive decline, affecting working memory, executive and visuospatial functions (Alcalay et al. 2012; Zokaei et al. 2014; Mata et al. 2016; Leocadi et al. 2022). The degree of cognitive impairment is correlated with the severity of GBA1 mutations (Petrucci et al. 2020; Szwedo et al. 2022). Deficient clearance mechanisms of a-synuclein in GBA1 mutants leading to accelerated a-synuclein pathology in cortical areas is thought to underlie the increased rates of cognitive impairment and ultimately, dementia in GBA1-PD patients (Jesus et al. 2016). Partial support exists for a subtle alteration in cognitive functioning in GBA1 mutation-positive individuals without PD (Avenali et al. 2019; Moran et al. 2021). Ongoing longitudinal studies aim to identify cognitive decline, other NMS and motor abnormalities in individuals harbouring GBA1 mutations prior to the onset of PD symptoms (Higgins et al. 2021). Data suggest that GBA1-PD has a stronger association with depression than iPD (Brockmann et al. 2011; Swan et al. 2016), albeit conflicting findings have been obtained (Zhang et al. 2015). Anxiety risk has been less extensively investigated in GBA1-PD, with only partial evidence supporting an increased risk in this patient cohort (Wang et al. 2014). Further, autonomic symptoms have been frequently reported in GBA1-PD patients including hyposmia, constipation, orthostatic hypotension and urogenital dysfunction (Brockmann et al. 2011).
Imaging
It is not currently possible to differentiate GBA1-PD from iPD based on neuroimaging alone (Barrett et al. 2013). Non-manifesting GBA1 mutation carrier status is not associated with reduced striatal dopaminergic tone (Goker-Alpan et al. 2012; Lopez et al. 2020; Simuni et al. 2020; Mullin et al. 2021). Utilising fluorodopa PET and transcranial sonography, no significant difference in nigrostriatal imaging has been reported between iPD and GBA1-PD patients (Kono et al. 2007; Kraoua et al. 2009; Goker-Alpan et al. 2012; Barrett et al. 2013; Lopez et al. 2020). Longitudinal investigations using single-photon emission computerised tomography (SPECT) and structural MRI have revealed a more accelerated disease course in GBA1-PD compared to iPD (Caminiti et al. 2022; Leocadi et al. 2022). Of note, iPD patients demonstrated similar patterns of cortical thinning to GBA1-PD 5 years post baseline MRI scans (Leocadi et al. 2022). The finding of a more aggressive progression in GBA1-PD cases has also been supported by cerebral blood flow (Goker-Alpan et al. 2012; Cilia et al. 2016) and FDG-PET studies (Greuel et al. 2020; Schindlbeck et al. 2020). Imaging in GBA1-PD has recently been reviewed (Filippi et al. 2022).
Neuropathology
Neuropathology studies of GBA1-PD brains have reported Lewy body aggregates in cortical regions, notably including the hippocampal regions CA2–4, in addition to the substantia nigra which is classically affected in iPD (Wong et al. 2004; Clark et al. 2009). Of note, others have not replicated such results (Parkkinen et al. 2011). Further reports indicate an increased rate of co-aggregation of GCase and a-synuclein in Lewy bodies in GBA1-PD brains (Goker-Alpan et al. 2010). Enhanced microglial activation in GBA1 mutation carriers without PD or dopaminergic loss has recently been reported (Mullin et al. 2021).
Mechanisms of GBA1-associated Parkinson disease
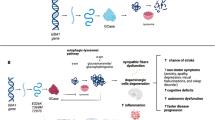
GCase activity is reduced to 58%, 67% and < 15% of normal function in GBA1-PD, iPD and GD patients, respectively (Gegg et al. 2012, Sidransky 2012). A minority of GD patients develop PD, indicating that PD risk is not proportional to GCase activity (Sidransky 2012). Numerous research efforts are underway to elicit pathways which may interact with GCase to increase PD risk in GBA1 mutation carriers, and whether such pathways are relevant to iPD patients. Proposed mechanisms include ER stress, autophagic lysosomal dysfunction, abnormal lipid homeostasis, mitochondrial dysfunction and neuroinflammation (Smith and Schapira 2022).
GBA1 gain-of-function mutations may lead to misfolded GCase being sequestered in the ER, impairing ER-associated degradation (ERAD) and triggering ER stress (Fernandes et al. 2016)). A-synuclein accumulation in the ER further impedes ER–Golgi transport (Cooper et al. 2006), upregulating the unfolded protein response (UPR) (Maor et al. 2013; Sanchez-Martinez et al. 2016). Induced pluripotent stem cell (iPSC)-derived neurones harbouring GBA1 mutations showed an increase in a-synuclein release following the activation of ER stress (Schondorf et al. 2014). Interestingly, GCase inhibitor, conduritol B epoxide (CBE), also produced cellular changes indicative of ER stress, providing support for both the loss and gain-of-function hypotheses in GBA1-PD pathogenesis (Kurzawa-Akanbi et al. 2012). Further, evaluations of GBA1-PD post-mortem brains have revealed altered levels of UPR-associated proteins, BiP, CHOP and HERP (Gegg et al. 2012; Kurzawa-Akanbi et al. 2012). GCase chaperone, Ambroxol, reversed the activation of UPR and was subsequently investigated as a disease-modifying therapy for PD in clinical trials (Suzuki et al. 2015b; Maor et al. 2016; Sanchez-Martinez et al. 2016). Moreover, it is noteworthy that ER stress may be an important therapeutic target in several neurodegenerative diseases (Kanekura et al. 2006; Yang et al. 2010; Liu et al. 2013).
GCase is involved in lysosomal clearance of a-synuclein (Mazzulli et al. 2011). GCase knockdown compromised lysosomal degradation of proteins, resulting in a 40% reduced rate of proteolysis (Mazzulli et al. 2011). Lysosomal/autophagic dysfunction contributes to the development of PD in GBA1 mutation carriers (Mazzulli et al. 2011; Magalhaes et al. 2016). Accumulation of a-synuclein and alterations in autophagy–lysosomal pathways occur concurrently in GCase deficient models (Cullen et al. 2011; Chiasserini et al. 2015; Rocha et al. 2015b). Autophagic impairment has also been noted in iPD brains (Alvarez-Erviti et al. 2010). A recent study reported that 50% of GCase in GBA-PD brain was present on the lysosomal surface and impaired chaperone-mediated autophagy, thereby increasing α-synuclein levels (Kuo et al. 2022).
GlcCer and GlcSph accumulation has been found in various GCase deficient models (Sardi et al. 2011; Xu et al. 2011; Farfel-Becker et al. 2014; Srikanth et al. 2021; Galvagnion et al. 2022). Serum ceramide, monohexosylceramide and sphingomyelin levels were raised in GBA1-PD patients compared to iPD (Guedes et al. 2017). GlcCer promotes the formation of proteinase K-resistant a-synuclein, suggesting it is involved in aggregating misfolded a-synuclein (Suzuki et al. 2015a). Lipids from GBA1-L444P fibroblasts accelerated a-synuclein aggregation compared to controls, with a-synuclein–lipid co-assembly indicating an active role for lipids in a-synuclein pathology (Galvagnion et al. 2022). Indeed, increased GlcSph levels are associated with increased substantia nigra p129/total α-synuclein ratios (Gundner et al. 2019). Ser129 phosphorylation of a-synuclein is present in > 90% of Lewy bodies, correlating with the progression of PD pathology (Fujiwara et al. 2002; Lue et al. 2012). Further, a link between lipid accumulation and worsening cognition in iPD patients has been suggested (Mielke et al. 2013). Normalising lipid levels in a GBA1-PD mouse model improved cognitive deficits (Sardi et al. 2017). However, whilst GlcCer and GlcSph accumulation has been observed in iPD and GD brains (Orvisky et al. 2002; Huebecker et al. 2020), it remains absent from the putamen and cerebellum of GBA1-PD patients (Gegg et al. 2012).
Mitochondrial dysfunction and oxidative stress play a key role in PD (Schapira et al. 1989). Reports of impaired mitochondrial function and morphology have emerged in numerous GCase deficient animal and cell models (Osellame et al. 2013; Garcia-Sanz et al. 2017; Li et al. 2019). Post CBE exposure, SHSY-5Y cells showed a progressive decline in mitochondrial membrane potential, increased free radical generation and a-synuclein levels (Cleeter et al. 2013). Similar findings were found in iPSC-derived neurones from GBA1-PD patients, albeit no gene dosage effect on mitochondrial function was observed between GBA1 heterozygous and knockout neurones (Schondorf et al. 2018). Different mechanisms may underlie the abnormal mitochondrial function according to GBA1 mutation number (Schondorf et al. 2018). Dysfunctional autophagy–lysosomal degradation pathways may contribute to the abnormal mitochondrial function observed in GCase deficient models (Schondorf et al. 2018). Further, L444P GBA1 knock-in mice displayed mitochondrial defects, with the concomitant a-synuclein accumulation in dopaminergic neurones rendering them more susceptible to the neurotoxin, MPTP (Yun et al. 2018).
Mounting evidence implicates neuroinflammation in PD pathogenesis, with elevated immune markers reported in the plasma (Miliukhina et al. 2020) and brain tissue (Mullin et al. 2021) isolated from GBA1-PD patients. Upregulation of complement C1q has been noted in the brains of mice following CBE exposure (Rocha et al. 2015b). GCase deficiency may increase the sensitivity of microglia to various insults, leading to an increased risk of neurodegeneration (Brunialti et al. 2021). Moreover, the finding of an association between HLA alleles and an increased risk of PD adds further support to the role of neuroinflammation in the disease process (International Parkinson’s Disease Genomics and Wellcome Trust Case Control 2011).
Potential GBA1-targeting therapies for PD
Enzyme replacement therapy and substrate reduction therapy
ERT and SRT has FDA approval for the treatment of GD. Preclinical work reported diminished a-synuclein pathology in human midbrain dopamine neurones post SRT administration (Zunke et al. 2018). However, the inability of such therapies to cross the BBB has limited its potential use. Alternative delivery methods to enhance neural GCase are under investigation (Poewe et al. 2017). Methods including BBB-penetrant peptides, exosome-mediated delivery and transport vehicle modified recombinant GCase may improve delivery of ERT (Gramlich et al. 2016; Hall et al. 2016; Ysselstein et al. 2021). Experimental BBB-penetrating SRT Venglustat was safe, well tolerated and demonstrated sufficient target engagement with a reduction in a-synuclein levels reported in the Phase I MOVES-PD clinical trial (Peterschmitt et al. 2022). A recent Phase II study of Venglustat revealed no such clinical benefit, with poorer performance in motor function observed following administration in a GBA1-PD cohort (Peterschmitt et al. 2021). However, it is known that specific GBA1 mutations affect the GCase protein differently, perhaps some therapies may be more beneficial when stratifying mutation carriers (Gan-Or et al. 2018).
Gene therapy
Replacement of mutant GBA1 gene via the adeno-associated virus (AAV) may be a therapeutic option in GBA1-PD (Hudry and Vandenberghe 2019). AAVs do not integrate into host genomes and possess a strong safety profile (Hudry and Vandenberghe 2019). AAV-GBA1 delivery in animal models has been reported to enhance GCase levels, reduce neuroinflammation and the accumulation of GlcSph, a-synuclein, ubiquitin and tau (Sardi et al. 2013; Rocha et al. 2015a; Massaro et al. 2018; Sucunza et al. 2021). Improved motor and memory function were also observed in mice and macaques following AAV-GBA1 administration (Sardi et al. 2013; Massaro et al. 2018). A Phase I/IIa clinical trial is currently underway to ascertain the safety, tolerability and clinical efficacy of AAV-GBA1 vector PR001 (LY3884961) in GBA1-PD patients and children with type 2 GD (ClinicalTrials.gov Identifier: NCT04127578 and NCT04411654, respectively).
Molecular chaperones
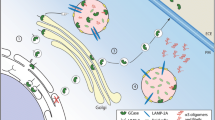
Chaperone therapy to disentangle misfolded GCase in ER and stabilise for transport to the lysosome is a promising therapeutic avenue in GBA1-PD (Fig. 1) (Ambrosi et al. 2015). The mucolytic ambroxol is a small molecule inhibitory chaperone capable of increasing GCase activity in mice, non-human primates and GBA1-PD patient cells (McNeill et al. 2014; Migdalska-Richards et al. 2016, 2017). Clinical trials are currently evaluating the efficacy of oral ambroxol in type 1 GD, PD dementia and GBA1-PD (ClinicalTrials.gov Identifier: NCT03950050, NCT02914366 and NCT02941822, respectively). Interestingly, ambroxol therapy achieved sufficient CSF penetration and target engagement, augmenting GCase protein levels (Mullin et al. 2020). Non-inhibitory chaperones, such as LTI-291, do not interact with an enzyme’s active site, and have been found to increase GCase activity and half-life in a phase I clinical trial (Trialregister.nl ID: NTR7299) (den Heijer et al. 2021).
The various approaches currently being explored in the search for neuroprotective therapies in GBA1-PD. Gene therapy may be used to replace the faulty GBA1 gene. Molecular chaperones may increase GCase activity by structurally correcting misfolded GCase. The use of enzyme replacement therapy (ERT) and substrate reduction therapy (SRT) to replenish GCase levels and reduce GlcCer accumulation in GBA1-PD, respectively, is being investigated
Conclusion
Significant insights into the pathogenesis of PD have been obtained following the finding of GBA1 mutations as the greatest numerical risk factor for PD. This link highlighted ER stress, lipid metabolism and autophagy–lysosomal pathways as key disease-causing mechanisms. GBA1-targeted therapies have been developed, with their clinical efficacy being evaluated in trials. It remains imperative to understand why only a minority of GBA1 mutation carriers progress to PD phenoconversion. Understanding more about the GBA1-PD link may help elucidate better treatments and methods of predicting who will develop the disease prior to symptom onset.
References
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R (2004) Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med 351:1972–1977. https://doi.org/10.1056/NEJMoa033277
Aharon-Peretz J, Badarny S, Rosenbaum H, Gershoni-Baruch R (2005) Mutations in the glucocerebrosidase gene and Parkinson disease: phenotype-genotype correlation. Neurology 65:1460–1461. https://doi.org/10.1212/01.wnl.0000176987.47875.28
Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, Fahn S, Dorovski T, Chung WK, Pauciulo M, Nichols W, Rana HQ, Balwani M, Bier L, Elstein D, Zimran A (2014) Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol 71:752–757. https://doi.org/10.1001/jamaneurol.2014.313
Alcalay RN, Wolf P, Chiang MSR, Helesicova K, Zhang XK, Merchant K, Hutten SJ, Scherzer C, Caspell-Garcia C, Blauwendraat C, Foroud T, Nudelman K, Gan-Or Z, Simuni T, Chahine LM, Levy O, Zheng D, Li G, Sardi SP, Parkinson’s Progression Markers, I (2020) Longitudinal measurements of glucocerebrosidase activity in Parkinson’s patients. Ann Clin Transl Neurol 7:1816–1830. https://doi.org/10.1002/acn3.51164
Alcalay, R. N., Caccappolo, E., Mejia-Santana, H., Tang, M., Rosado, L., Orbe Reilly, M., Ruiz, D., Ross, B., Verbitsky, M., Kisselev, S., Louis, E., Comella, C., Colcher, A., Jennings, D., Nance, M., Bressman, S., Scott, W. K., Tanner, C., Mickel, S., Andrews, H., Waters, C., Fahn, S., Cote, L., Frucht, S., Ford, B., Rezak, M., Novak, K., Friedman, J. H., Pfeiffer, R., Marsh, L., Hiner, B., Siderowf, A., Payami, H., Molho, E., Factor, S., Ottman, R., Clark, L. N. & Marder, K. (2012) Cognitive performance of GBA mutation carriers with early-onset PD: the CORE-PD study. Neurology 78:1434-40. https://doi.org/10.1212/WNL.0b013e318253d54b
Alfonso P, Rodriguez-Rey JC, Ganan A, Perez-Calvo JI, Giralt M, Giraldo P, Pocovi M (2004) Expression and functional characterization of mutated glucocerebrosidase alleles causing Gaucher disease in Spanish patients. Blood Cells Mol Dis 32:218–225. https://doi.org/10.1016/j.bcmd.2003.10.010
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 67:1464–1472. https://doi.org/10.1001/archneurol.2010.198
Ambrosi G, Ghezzi C, Zangaglia R, Levandis G, Pacchetti C, Blandini F (2015) Ambroxol-induced rescue of defective glucocerebrosidase is associated with increased LIMP-2 and saposin C levels in GBA1 mutant Parkinson’s disease cells. Neurobiol Dis 82:235–242. https://doi.org/10.1016/j.nbd.2015.06.008
Anheim M, Elbaz A, Lesage S, Durr A, Condroyer C, Viallet F, Pollak P, Bonaiti B, Bonaiti-Pellie C, Brice A, French Parkinson Disease Genetic, G (2012) Penetrance of Parkinson disease in glucocerebrosidase gene mutation carriers. Neurology 78:417–420. https://doi.org/10.1212/WNL.0b013e318245f476
Asselta R, Rimoldi V, Siri C, Cilia R, Guella I, Tesei S, Solda G, Pezzoli G, Duga S, Goldwurm S (2014) Glucocerebrosidase mutations in primary Parkinsonism. Parkinsonism Relat Disord 20:1215–1220. https://doi.org/10.1016/j.parkreldis.2014.09.003
Avenali M, Toffoli M, Mullin S, McNeil A, Hughes DA, Mehta A, Blandini F, Schapira AHV (2019) Evolution of prodromal parkinsonian features in a cohort of GBA mutation-positive individuals: a 6-year longitudinal study. J Neurol Neurosurg Psychiatry 90:1091–1097. https://doi.org/10.1136/jnnp-2019-320394
Avenali M, Cerri S, Ongari G, Ghezzi C, Pacchetti C, Tassorelli C, Valente EM, Blandini F (2021) Profiling the biochemical signature of GBA-related Parkinson’s disease in peripheral blood mononuclear cells. Mov Disord 36:1267–1272. https://doi.org/10.1002/mds.28496
Balestrino R, Tunesi S, Tesei S, Lopiano L, Zecchinelli AL, Goldwurm S (2020) Penetrance of glucocerebrosidase (GBA) mutations in Parkinson’s disease: a Kin cohort study. Mov Disord 35:2111–2114. https://doi.org/10.1002/mds.28200
Barrett MJ, Hagenah J, Dhawan V, Peng S, Stanley K, Raymond D, Deik A, Gross SJ, Schreiber-Agus N, Mirelman A, Marder K, Ozelius LJ, Eidelberg D, Bressman SB, Saunders-Pullman R, Consortium, L. A. J. (2013) Transcranial sonography and functional imaging in glucocerebrosidase mutation Parkinson disease. Parkinsonism Relat Disord 19:186–91. https://doi.org/10.1016/j.parkreldis.2012.09.007
Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH (2015) Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation-positive cohort. JAMA Neurol 72:201–208. https://doi.org/10.1001/jamaneurol.2014.2950
Brady RO, Kanfer J, Shapiro D (1965) The metabolism of glucocerebrosides. I. Purification and properties of a glucocerebroside-cleaving enzyme from spleen tissue. J Biol Chem 240:39–43
Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, Berg D (2011) GBA-associated PD presents with nonmotor characteristics. Neurology 77:276–280. https://doi.org/10.1212/WNL.0b013e318225ab77
Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, Gasser T, Berg D (2015) GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord 30:407–411. https://doi.org/10.1002/mds.26071
Brunialti E, Villa A, Mekhaeil M, Mornata F, Vegeto E, Maggi A, di Monte DA, Ciana P (2021) Inhibition of microglial beta-glucocerebrosidase hampers the microglia-mediated antioxidant and protective response in neurons. J Neuroinflammation 18:220. https://doi.org/10.1186/s12974-021-02272-2
Caminiti SP, Carli G, Avenali M, Blandini F, Perani D (2022) Clinical and dopamine transporter imaging trajectories in a cohort of Parkinson’s disease patients with GBA mutations. Mov Disord 37:106–118. https://doi.org/10.1002/mds.28818
Chen J, Li W, Zhang T, Wang YJ, Jiang XJ, Xu ZQ (2014) Glucocerebrosidase gene mutations associated with Parkinson’s disease: a meta-analysis in a Chinese population. PLoS ONE 9:e115747. https://doi.org/10.1371/journal.pone.0115747
Chiasserini D, Paciotti S, Eusebi P, Persichetti E, Tasegian A, Kurzawa-Akanbi M, Chinnery PF, Morris CM, Calabresi P, Parnetti L, Beccari T (2015) Selective loss of glucocerebrosidase activity in sporadic Parkinson’s disease and dementia with Lewy bodies. Mol Neurodegener 10:15. https://doi.org/10.1186/s13024-015-0010-2
Cilia R, Tunesi S, Marotta G, Cereda E, Siri C, Tesei S, Zecchinelli AL, Canesi M, Mariani CB, Meucci N, Sacilotto G, Zini M, Barichella M, Magnani C, Duga S, Asselta R, Solda G, Seresini A, Seia M, Pezzoli G, Goldwurm S (2016) Survival and dementia in GBA-associated Parkinson’s disease: the mutation matters. Ann Neurol 80:662–673. https://doi.org/10.1002/ana.24777
Clark LN, Kartsaklis LA, Wolf Gilbert R, Dorado B, Ross BM, Kisselev S, Verbitsky M, Mejia-Santana H, Cote LJ, Andrews H, Vonsattel JP, Fahn S, Mayeux R, Honig LS, Marder K (2009) Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol 66:578–83. https://doi.org/10.1001/archneurol.2009.54
Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH (2013) Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int 62:1–7. https://doi.org/10.1016/j.neuint.2012.10.010
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313:324–328. https://doi.org/10.1126/science.1129462
Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, Rochet JC, Glicksman MA, Cheng SH, Grabowski GA, Shihabuddin LS, Schlossmacher MG (2011) Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol 69:940–953. https://doi.org/10.1002/ana.22400
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/s0896-6273(03)00568-3
den Heijer JM, Kruithof AC, van Amerongen G, de Kam ML, Thijssen E, Grievink HW, Moerland M, Walker M, Been K, Skerlj R, Justman C, Dudgeon L, Lansbury P, Cullen VC, Hilt DC, Groeneveld GJ (2021) A randomized single and multiple ascending dose study in healthy volunteers of LTI-291, a centrally penetrant glucocerebrosidase activator. Br J Clin Pharmacol. https://doi.org/10.1111/bcp.14772
Duran R, Mencacci NE, Angeli AV, Shoai M, Deas E, Houlden H, Mehta A, Hughes D, Cox TM, Deegan P, Schapira AH, Lees AJ, Limousin P, Jarman PR, Bhatia KP, Wood NW, Hardy J, Foltynie T (2013) The glucocerobrosidase E326K variant predisposes to Parkinson’s disease, but does not cause Gaucher’s disease. Mov Disord 28:232–236. https://doi.org/10.1002/mds.25248
Fan K, Tang BS, Wang YQ, Kang JF, Li K, Liu ZH, Sun QY, Xu Q, Yan XX, Guo JF (2016) The GBA, DYRK1A and MS4A6A polymorphisms influence the age at onset of Chinese Parkinson patients. Neurosci Lett 621:133–136. https://doi.org/10.1016/j.neulet.2016.04.014
Farfel-Becker, T., Vitner, E. B., Kelly, S. L., Bame, J. R., Duan, J., Shinder, V., Merrill, A. H., JR., Dobrenis, K. & Futerman, A. H. (2014) Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum Mol Genet 23: 843-54. https://doi.org/10.1093/hmg/ddt468
Fereshtehnejad SM, Yao C, Pelletier A, Montplaisir JY, Gagnon JF, Postuma RB (2019) Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 142:2051–2067. https://doi.org/10.1093/brain/awz111
Fernandes HJ, Hartfield EM, Christian HC, Emmanoulidou E, Zheng Y, Booth H, Bogetofte H, Lang C, Ryan BJ, Sardi SP, Badger J, Vowles J, Evetts S, Tofaris GK, Vekrellis K, Talbot K, Hu MT, James W, Cowley SA, Wade-Martins R (2016) ER stress and autophagic perturbations lead to elevated extracellular alpha-synuclein in GBA-N370S Parkinson’s iPSC-derived dopamine neurons. Stem Cell Reports 6:342–356. https://doi.org/10.1016/j.stemcr.2016.01.013
Filippi M, Balestrino R, Basaia S, Agosta F (2022) Neuroimaging in glucocerebrosidase-associated Parkinsonism: a systematic review. Mov Disord. https://doi.org/10.1002/mds.29047
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T (2002) Alpha-synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol 4:160–164. https://doi.org/10.1038/ncb748
Galvagnion C, Marlet FR, Cerri S, Schapira AHV, Blandini F, di Monte DA (2022) Sphingolipid changes in Parkinson L444P GBA mutation fibroblasts promote alpha-synuclein aggregation. Brain 145:1038–1051. https://doi.org/10.1093/brain/awab371
Gan-Or Z, Liong C, Alcalay RN (2018) GBA-associated Parkinson’s disease and other synucleinopathies. Curr Neurol Neurosci Rep 18:44. https://doi.org/10.1007/s11910-018-0860-4
Garcia-Sanz P, Orgaz L, Bueno-Gil G, Espadas I, Rodriguez-Traver E, Kulisevsky J, Gutierrez A, Davila JC, Gonzalez-Polo RA, Fuentes JM, Mir P, Vicario C, Moratalla R (2017) N370S-GBA1 mutation causes lysosomal cholesterol accumulation in Parkinson’s disease. Mov Disord 32:1409–1422. https://doi.org/10.1002/mds.27119
Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH (2012) Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann Neurol 72:455–463. https://doi.org/10.1002/ana.23614
Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E (2003) Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J Pediatr 143:273–276. https://doi.org/10.1067/S0022-3476(03)00302-0
Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E (2010) Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol 120:641–649. https://doi.org/10.1007/s00401-010-0741-7
Goker-Alpan O, Masdeu JC, Kohn PD, Ianni A, Lopez G, Groden C, Chapman MC, Cropp B, Eisenberg DP, Maniwang ED, Davis J, Wiggs E, Sidransky E, Berman KF (2012) The neurobiology of glucocerebrosidase-associated Parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain 135:2440–2448. https://doi.org/10.1093/brain/aws174
Gomez G, Arias S, Cardenas L, Zoghbi D, Paradisi I (2017) GBA mutations in Gaucher type I Venezuelan patients: ethnic origins and frequencies. J Genet 96:583–589. https://doi.org/10.1007/s12041-017-0821-8
Grabowski GA (2008) Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 372:1263–71. https://doi.org/10.1016/S0140-6736(08)61522-6
Grabowski GA, Horowitz M (1997) Gaucher’s disease: molecular, genetic and enzymological aspects. Baillieres Clin Haematol 10:635–656. https://doi.org/10.1016/s0950-3536(97)80032-7
Grabowski GA, Gatt S, Horowitz M (1990) Acid beta-glucosidase: enzymology and molecular biology of Gaucher disease. Crit Rev Biochem Mol Biol 25:385–414. https://doi.org/10.3109/10409239009090616
Grabowski GA, Zimran A, Ida H (2015) Gaucher disease types 1 and 3: phenotypic characterization of large populations from the ICGG Gaucher registry. Am J Hematol 90(Suppl 1):S12–S18. https://doi.org/10.1002/ajh.24063
Gramlich PA, Westbroek W, Feldman RA, Awad O, Mello N, Remington MP, Sun Y, Zhang W, Sidransky E, Betenbaugh MJ, Fishman PS (2016) A peptide-linked recombinant glucocerebrosidase for targeted neuronal delivery: design, production, and assessment. J Biotechnol 221:1–12. https://doi.org/10.1016/j.jbiotec.2016.01.015
Greuel A, Trezzi JP, Glaab E, Ruppert MC, Maier F, Jager C, Hodak Z, Lohmann K, Ma Y, Eidelberg D, Timmermann L, Hiller K, Tittgemeyer M, Drzezga A, Diederich N, Eggers C (2020) GBA variants in Parkinson’s disease: clinical, metabolomic, and multimodal neuroimaging phenotypes. Mov Disord 35:2201–2210. https://doi.org/10.1002/mds.28225
Guedes LC, Chan RB, Gomes MA, Conceicao VA, Machado RB, Soares T, Xu Y, Gaspar P, Carrico JA, Alcalay RN, Ferreira JJ, Outeiro TF, Miltenberger-Miltenyi G (2017) Serum lipid alterations in GBA-associated Parkinson’s disease. Parkinsonism Relat Disord 44:58–65. https://doi.org/10.1016/j.parkreldis.2017.08.026
Gundner AL, Duran-Pacheco G, Zimmermann S, Ruf I, Moors T, Baumann K, Jagasia R, van de Berg WDJ, Kremer T (2019) Path mediation analysis reveals GBA impacts Lewy body disease status by increasing alpha-synuclein levels. Neurobiol Dis 121:205–213. https://doi.org/10.1016/j.nbd.2018.09.015
Guo JF, Li K, Yu RL, Sun QY, Wang L, Yao LY, Hu YC, Lv ZY, Luo LZ, Shen L, Jiang H, Yan XX, Pan Q, Xia K, Tang BS (2015) Polygenic determinants of Parkinson’s disease in a Chinese population. Neurobiol Aging 36:17651–17656. https://doi.org/10.1016/j.neurobiolaging.2014.12.030
Gustafsson H, Nordstrom A, Nordstrom P (2015) Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 84:2422–2429. https://doi.org/10.1212/WNL.0000000000001684
Hall J, Prabhakar S, Balaj L, Lai CP, Cerione RA, Breakefield XO (2016) Delivery of therapeutic proteins via extracellular vesicles: review and potential treatments for Parkinson’s disease, Glioma, and Schwannoma. Cell Mol Neurobiol 36:417–427. https://doi.org/10.1007/s10571-015-0309-0
Higgins AL, Toffoli M, Mullin S, Lee CY, Koletsi S, Avenali M, Blandini F, Schapira AH (2021) The remote assessment of parkinsonism supporting the ongoing development of interventions in Gaucher disease. Neurodegener Dis Manag 11:451–458. https://doi.org/10.2217/nmt-2021-0032
Hruska KS, Lamarca ME, Scott CR, Sidransky E (2008) Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 29:567–583. https://doi.org/10.1002/humu.20676
Hu FY, Xi J, Guo J, Yu LH, Liu L, He XH, Liu ZL, Zou XY, Xu YM (2010) Association of the glucocerebrosidase N370S allele with Parkinson’s disease in two separate Chinese Han populations of mainland China. Eur J Neurol 17:1476–1478. https://doi.org/10.1111/j.1468-1331.2010.03097.x
Hudry E, Vandenberghe LH (2019) Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron 101:839–862. https://doi.org/10.1016/j.neuron.2019.02.017
Huebecker M, Moloney EB, van der Spoel AC, Priestman DA, Isacson O, Hallett PJ, Platt FM (2020) Correction to: Reduced sphingolipid hydrolase activities, substrate accumulation and ganglioside decline in Parkinson’s disease. Mol Neurodegener 15:6. https://doi.org/10.1186/s13024-019-0355-z
INTERNATIONAL PARKINSON'S DISEASE GENOMICS, C., WELLCOME TRUST CASE CONTROL, C. (2011) A two-stage meta-analysis identifies several new loci for Parkinson's disease. PLoS Genet 7:e1002142. https://doi.org/10.1371/journal.pgen.1002142
Jesus S, Huertas I, Bernal-Bernal I, Bonilla-Toribio M, Caceres-Redondo MT, Vargas-Gonzalez L, Gomez-Llamas M, Carrillo F, Calderon E, Carballo M, Gomez-Garre P, Mir P (2016) GBA variants influence motor and non-motor features of Parkinson’s disease. PLoS ONE 11:e0167749. https://doi.org/10.1371/journal.pone.0167749
Jung O, Patnaik S, Marugan J, Sidransky E, Westbroek W (2016) Progress and potential of non-inhibitory small molecule chaperones for the treatment of Gaucher disease and its implications for Parkinson disease. Expert Rev Proteomics 13:471–479. https://doi.org/10.1080/14789450.2016.1174583
Kacher Y, Brumshtein B, Boldin-Adamsky S, Toker L, Shainskaya A, Silman I, Sussman JL, Futerman AH (2008) Acid beta-glucosidase: insights from structural analysis and relevance to Gaucher disease therapy. Biol Chem 389:1361–1369. https://doi.org/10.1515/BC.2008.163
Kanekura K, Nishimoto I, Aiso S, Matsuoka M (2006) Characterization of amyotrophic lateral sclerosis-linked P56S mutation of vesicle-associated membrane protein-associated protein B (VAPB/ALS8). J Biol Chem 281:30223–30233. https://doi.org/10.1074/jbc.M605049200
Kono S, Shirakawa K, Ouchi Y, Sakamoto M, Ida H, Sugiura T, Tomiyama H, Suzuki H, Takahashi Y, Miyajima H, Hattori N, Mizuno Y (2007) Dopaminergic neuronal dysfunction associated with parkinsonism in both a Gaucher disease patient and a carrier. J Neurol Sci 252:181–184. https://doi.org/10.1016/j.jns.2006.10.019
Koprivica V, Stone DL, Park JK, Callahan M, Frisch A, Cohen IJ, Tayebi N, Sidransky E (2000) Analysis and classification of 304 mutant alleles in patients with type 1 and type 3 Gaucher disease. Am J Hum Genet 66:1777–1786. https://doi.org/10.1086/302925
Kraoua I, Stirnemann J, Ribeiro MJ, Rouaud T, Verin M, Annic A, Rose C, Defebvre L, Remenieras L, Schupbach M, Belmatoug N, Vidailhet M, Sedel F (2009) Parkinsonism in Gaucher’s disease type 1: ten new cases and a review of the literature. Mov Disord 24:1524–1530. https://doi.org/10.1002/mds.22593
Kuo SH, Tasset I, Cheng MM, Diaz A, Pan MK, Lieberman OJ, Hutten SJ, Alcalay RN, Kim S, Ximenez-Embun P, Fan L, Kim D, Ko HS, Yacoubian T, Kanter E, Liu L, Tang G, Munoz J, Sardi SP, Li A, Gan L, Cuervo AM, Sulzer D (2022) Mutant glucocerebrosidase impairs alpha-synuclein degradation by blockade of chaperone-mediated autophagy. Sci Adv 8:eabm6393. https://doi.org/10.1126/sciadv.abm6393
Kurzawa-Akanbi M, Hanson PS, Blain PG, Lett DJ, McKeith IG, Chinnery PF, Morris CM (2012) Glucocerebrosidase mutations alter the endoplasmic reticulum and lysosomes in Lewy body disease. J Neurochem 123:298–309. https://doi.org/10.1111/j.1471-4159.2012.07879.x
Leocadi M, Canu E, Donzuso G, Stojkovic T, Basaia S, Kresojevic N, Stankovic I, Sarasso E, Piramide N, Tomic A, Markovic V, Petrovic I, Stefanova E, Kostic VS, Filippi M, Agosta F (2022) Longitudinal clinical, cognitive, and neuroanatomical changes over 5 years in GBA-positive Parkinson’s disease patients. J Neurol 269:1485–1500. https://doi.org/10.1007/s00415-021-10713-4
Lesage S, Anheim M, Condroyer C, Pollak P, Durif F, Dupuits C, Viallet F, Lohmann E, Corvol JC, Honore A, Rivaud S, Vidailhet M, Durr A, Brice A, FRENCH PARKINSON’S DISEASE GENETICS STUDY, G (2011) Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet 20:202–210. https://doi.org/10.1093/hmg/ddq454
Li H, Ham A, Ma TC, Kuo SH, Kanter E, Kim D, Ko HS, Quan Y, Sardi SP, Li A, Arancio O, Kang UJ, Sulzer D, Tang G (2019) Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy 15:113–130. https://doi.org/10.1080/15548627.2018.1509818
Liu SY, Wang W, Cai ZY, Yao LF, Chen ZW, Wang CY, Zhao B, Li KS (2013) Polymorphism -116C/G of human X-box-binding protein 1 promoter is associated with risk of Alzheimer’s disease. CNS Neurosci Ther 19:229–234. https://doi.org/10.1111/cns.12064
Lopez G, Eisenberg DP, Gregory MD, Ianni AM, Grogans SE, Masdeu JC, Kim J, Groden C, Sidransky E, Berman KF (2020) Longitudinal positron emission tomography of dopamine synthesis in subjects with GBA1 mutations. Ann Neurol 87:652–657. https://doi.org/10.1002/ana.25692
Lue LF, Walker DG, Adler CH, Shill H, Tran H, Akiyama H, Sue LI, Caviness J, Sabbagh MN, Beach TG (2012) Biochemical increase in phosphorylated alpha-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol 22:745–756. https://doi.org/10.1111/j.1750-3639.2012.00585.x
Lukina E, Watman N, Dragosky M, Lau H, Avila Arreguin E, Rosenbaum H, Zimran A, Foster MC, Gaemers SJM, Peterschmitt MJ (2019) Outcomes after 8 years of eliglustat therapy for Gaucher disease type 1: final results from the Phase 2 trial. Am J Hematol 94:29–38. https://doi.org/10.1002/ajh.25300
Machaczka M, Rucinska M, Skotnicki AB, Jurczak W (1999) Parkinson’s syndrome preceding clinical manifestation of Gaucher’s disease. Am J Hematol 61:216–217. https://doi.org/10.1002/(sici)1096-8652(199907)61:3%3c216::aid-ajh12%3e3.0.co;2-b
Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AH (2016) Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet 25:3432–3445. https://doi.org/10.1093/hmg/ddw185
Malini E, Grossi S, Deganuto M, Rosano C, Parini R, Dominisini S, Cariati R, Zampieri S, Bembi B, Filocamo M, Dardis A (2014) Functional analysis of 11 novel GBA alleles. Eur J Hum Genet 22:511–516. https://doi.org/10.1038/ejhg.2013.182
Maor G, Rencus-Lazar S, Filocamo M, Steller H, Segal D, Horowitz M (2013) Unfolded protein response in Gaucher disease: from human to Drosophila. Orphanet J Rare Dis 8:140. https://doi.org/10.1186/1750-1172-8-140
Maor G, Cabasso O, Krivoruk O, Rodriguez J, Steller H, Segal D, Horowitz M (2016) The contribution of mutant GBA to the development of Parkinson disease in Drosophila. Hum Mol Genet 25:2712–2727. https://doi.org/10.1093/hmg/ddw129
Massaro G, Mattar CNZ, Wong AMS, Sirka E, Buckley SMK, Herbert BR, Karlsson S, Perocheau DP, Burke D, Heales S, Richard-Londt A, Brandner S, Huebecker M, Priestman DA, Platt FM, Mills K, Biswas A, Cooper JD, Chan JKY, Cheng SH, Waddington SN, Rahim AA (2018) Fetal gene therapy for neurodegenerative disease of infants. Nat Med 24:1317–1323. https://doi.org/10.1038/s41591-018-0106-7
Mata IF, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, van Deerlin VM, Ritz B, Rausch R, Factor SA, Wood-Siverio C, Quinn JF, Chung KA, Peterson-Hiller AL, Goldman JG, Stebbins GT, Bernard B, Espay AJ, Revilla FJ, Devoto J, Rosenthal LS, Dawson TM, Albert MS, Tsuang D, Huston H, Yearout D, Hu SC, Cholerton BA, Montine TJ, Edwards KL, Zabetian CP (2016) GBA Variants are associated with a distinct pattern of cognitive deficits in Parkinson’s disease. Mov Disord 31:95–102. https://doi.org/10.1002/mds.26359
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D (2011) Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146:37–52. https://doi.org/10.1016/j.cell.2011.06.001
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology 89:88–100. https://doi.org/10.1212/WNL.0000000000004058
McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH (2014) Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 137:1481–1495. https://doi.org/10.1093/brain/awu020
Mielke MM, Maetzler W, Haughey NJ, Bandaru VV, Savica R, Deuschle C, Gasser T, Hauser AK, Graber-Sultan S, Schleicher E, Berg D, Liepelt-Scarfone I (2013) Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: a pilot study. PLoS ONE 8:e73094. https://doi.org/10.1371/journal.pone.0073094
Migdalska-Richards A, Daly L, Bezard E, Schapira AH (2016) Ambroxol effects in glucocerebrosidase and alpha-synuclein transgenic mice. Ann Neurol 80:766–775. https://doi.org/10.1002/ana.24790
Migdalska-Richards A, Ko WKD, Li Q, Bezard E, Schapira AHV (2017) Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse. https://doi.org/10.1002/syn.21967
Miliukhina IV, Usenko TS, Senkevich KA, Nikolaev MA, Timofeeva AA, Agapova EA, Semenov AV, Lubimova NE, Totolyan AA, Pchelina SN (2020) Plasma cytokines profile in patients with Parkinson’s disease associated with mutations in GBA gene. Bull Exp Biol Med 168:423–426. https://doi.org/10.1007/s10517-020-04723-x
Mistry PK, Cappellini MD, Lukina E, Ozsan H, Mach Pascual S, Rosenbaum H, Helena Solano M, Spigelman Z, Villarrubia J, Watman NP, Massenkeil G (2011) A reappraisal of Gaucher disease-diagnosis and disease management algorithms. Am J Hematol 86:110–5. https://doi.org/10.1002/ajh.21888
Mistry PK, Belmatoug N, vom Dahl S, Giugliani R (2015) Understanding the natural history of Gaucher disease. Am J Hematol 90(Suppl 1):S6-11. https://doi.org/10.1002/ajh.24055
Moran EE, Bressman SB, Ortega RA, Raymond D, Nichols WC, Palmese CA, Elango S, Swan M, Shanker V, Perera I, Wang C, Zimmerman ME, Saunders-Pullman R (2021) Cognitive Functioning of Glucocerebrosidase (GBA) Non-manifesting Carriers. Front Neurol 12:635958. https://doi.org/10.3389/fneur.2021.635958
Mullin S, Smith L, Lee K, D’Souza G, Woodgate P, Elflein J, Hallqvist J, Toffoli M, Streeter A, Hosking J, Heywood WE, Khengar R, Campbell P, Hehir J, Cable S, Mills K, Zetterberg H, Limousin P, Libri V, Foltynie T, Schapira AHV (2020) Ambroxol for the Treatment of Patients With Parkinson Disease With and Without Glucocerebrosidase Gene Mutations: A Nonrandomized, Noncontrolled Trial. JAMA Neurol 77:427–434. https://doi.org/10.1001/jamaneurol.2019.4611
Mullin S, Stokholm MG, Hughes D, Mehta A, Parbo P, Hinz R, Pavese N, Brooks DJ, Schapira AHV (2021) Brain microglial activation increased in glucocerebrosidase (GBA) mutation carriers without Parkinson’s disease. Mov Disord 36:774–779. https://doi.org/10.1002/mds.28375
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, Destefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, International Parkinson’s Disease Genomics, C., Parkinson’s Study Group Parkinson’s Research: The Organized, G. I., Andme, Genepd, Neurogenetics Research, C., Hussman Institute Of Human, G., Ashkenazi Jewish Dataset, I., Cohorts For, H., Aging Research IN Genetic, E., North American Brain Expression, C., United Kingdom Brain Expression, C., Greek Parkinson’s Disease, C., Alzheimer Genetic Analysis, G. (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46:989–93. https://doi.org/10.1038/ng.3043
Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A (1996) Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM 89:691–694. https://doi.org/10.1093/qjmed/89.9.691
Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132:1783–1794. https://doi.org/10.1093/brain/awp044
Omer N, Giladi N, Gurevich T, Bar-Shira A, Gana-Weisz M, Glinka T, Goldstein O, Kestenbaum M, Cedarbaum JM, Mabrouk OS, Fraser KB, Shirvan JC, Orr-Urtreger A, Mirelman A, Thaler A (2022) Glucocerebrosidase Activity is not Associated with Parkinson’s Disease Risk or Severity. Mov Disord 37:190–195. https://doi.org/10.1002/mds.28792
O’Regan G, Desouza RM, Balestrino R, Schapira AH (2017) Glucocerebrosidase Mutations in Parkinson Disease. J Parkinsons Dis 7:411–422. https://doi.org/10.3233/JPD-171092
Orvisky E, Park JK, Lamarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E (2002) Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol Genet Metab 76:262–270. https://doi.org/10.1016/s1096-7192(02)00117-8
Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AHV, Duchen MR (2013) Mitochondria and quality control defects in a mouse model of Gaucher disease–links to Parkinson’s disease. Cell Metab 17:941–953. https://doi.org/10.1016/j.cmet.2013.04.014
Parenti G (2009) Treating lysosomal storage diseases with pharmacological chaperones: from concept to clinics. EMBO Mol Med 1:268–79. https://doi.org/10.1002/emmm.200900036
Parkkinen L, Neumann J, O’Sullivan SS, Holton JL, Revesz T, Hardy J, Lees AJ (2011) Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson’s disease. Mol Genet Metab 103:410–412. https://doi.org/10.1016/j.ymgme.2011.04.015
Pellicano C, Benincasa D, Pisani V, Buttarelli FR, Giovannelli M, Pontieri FE (2007) Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatr Dis Treat 3:145–152. https://doi.org/10.2147/nedt.2007.3.1.145
Peterschmitt MJ, Saiki H, Hatano T, Gasser T, Isaacson SH, Gaemers SJM, Minini P, Saubadu S, Sharma J, Walbillic S, Alcalay RN, Cutter G, Hattori N, Hoglinger GU, Marek K, Schapira AHV, Scherzer CR, Simuni T, Giladi N, Sardi SP, Fischer TZ, Investigators M-P (2022) Safety, pharmacokinetics, and pharmacodynamics of oral venglustat in patients with Parkinson’s disease and a GBA mutation: results from part 1 of the randomized, double-blinded, placebo-controlled MOVES-PD trial. J Parkinsons Dis 12:557–570. https://doi.org/10.3233/JPD-212714
Peterschmitt, J., Giladi, N., Alcalay, R., Cutter, G., Höglinger, G., Schapira, A., Scherzer, C., Simuni, T., Gurevich, T., Gasser, T., Pacchetti, C., Marek, K., Minini, P. & Sardi, S. (2021) Effect of venglustat by GBA mutation severity in patients with Parkinson’s disease [abstract]. Mov Disord 36.
Petrucci S, Ginevrino M, Trezzi I, Monfrini E, Ricciardi L, Albanese A, Avenali M, Barone P, Bentivoglio AR, Bonifati V, Bove F, Bonanni L, Brusa L, Cereda C, Cossu G, Criscuolo C, Dati G, de Rosa A, Eleopra R, Fabbrini G, Fadda L, Garbellini M, Minafra B, Onofrj M, Pacchetti C, Palmieri I, Pellecchia MT, Petracca M, Picillo M, Pisani A, Vallelunga A, Zangaglia R, di Fonzo A, Morgante F, Valente EM, GROUP, I.-G.-P. S (2020) GBA-related Parkinson’s disease: dissection of genotype-phenotype correlates in a large Italian cohort. Mov Disord 35:2106–2111. https://doi.org/10.1002/mds.28195
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag AE, Lang AE (2017) Parkinson disease. Nat Rev Dis Primers 3:17013. https://doi.org/10.1038/nrdp.2017.13
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Pringsheim T, Jette N, Frolkis A, Steeves TD (2014) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29:1583–1590. https://doi.org/10.1002/mds.25945
Rana HQ, Balwani M, Bier L, Alcalay RN (2013) Age-specific Parkinson disease risk in GBA mutation carriers: information for genetic counseling. Genet Med 15:146–149. https://doi.org/10.1038/gim.2012.107
Riboldi GM, di Fonzo AB (2019) GBA, Gaucher disease, and Parkinson’s disease: from genetic to clinic to new therapeutic approaches. Cells. https://doi.org/10.3390/cells8040364
Robak LA, Jansen IE, van Rooij J, Uitterlinden AG, Kraaij R, Jankovic J, Heutink P, Shulman JM, International Parkinson’s Disease Genomics, C. (2017) Excessive burden of lysosomal storage disorder gene variants in Parkinson’s disease. Brain 140:3191–3203. https://doi.org/10.1093/brain/awx285
Rocha EM, Smith GA, Park E, Cao H, Brown E, Hayes MA, Beagan J, McLean JR, Izen SC, Perez-Torres E, Hallett PJ, Isacson O (2015a) Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons. Neurobiol Dis 82:495–503. https://doi.org/10.1016/j.nbd.2015.09.009
Rocha EM, Smith GA, Park E, Cao H, Graham AR, Brown E, McLean JR, Hayes MA, Beagan J, Izen SC, Perez-Torres E, Hallett PJ, Isacson O (2015b) Sustained systemic glucocerebrosidase inhibition induces brain alpha-synuclein aggregation, microglia and complement C1q activation in mice. Antioxid Redox Signal 23:550–564. https://doi.org/10.1089/ars.2015.6307
Rosenbloom BE, Weinreb NJ (2013) Gaucher disease: a comprehensive review. Crit Rev Oncog 18:163–175. https://doi.org/10.1615/critrevoncog.2013006060
Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR (2004) Parkinsonian signs and substantia nigra neuron density in decendents elders without PD. Ann Neurol 56:532–539. https://doi.org/10.1002/ana.20226
Sanchez-Martinez A, Beavan M, Gegg ME, Chau KY, Whitworth AJ, Schapira AH (2016) Parkinson disease-linked GBA mutation effects reversed by molecular chaperones in human cell and fly models. Sci Rep 6:31380. https://doi.org/10.1038/srep31380
Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, Passini MA, Grabowski GA, Schlossmacher MG, Sidman RL, Cheng SH, Shihabuddin LS (2011) CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci U S A 108:12101–12106. https://doi.org/10.1073/pnas.1108197108
Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, Bu J, Sweet L, Passini MA, Dodge JC, Yu WH, Sidman RL, Cheng SH, Shihabuddin LS (2013) Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci USA 110:3537–3542. https://doi.org/10.1073/pnas.1220464110
Sardi SP, Viel C, Clarke J, Treleaven CM, Richards AM, Park H, Olszewski MA, Dodge JC, Marshall J, Makino E, Wang B, Sidman RL, Cheng SH, Shihabuddin LS (2017) Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc Natl Acad Sci USA 114:2699–2704. https://doi.org/10.1073/pnas.1616152114
Schapira AH (2015) Glucocerebrosidase and Parkinson disease: recent advances. Mol Cell Neurosci 66:37–42. https://doi.org/10.1016/j.mcn.2015.03.013
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269. https://doi.org/10.1016/s0140-6736(89)92366-0
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18:435–450. https://doi.org/10.1038/nrn.2017.62
Schindlbeck KA, Vo A, Nguyen N, Tang CC, Niethammer M, Dhawan V, Brandt V, Saunders-Pullman R, Bressman SB, Eidelberg D (2020) LRRK2 and GBA variants exert distinct influences on Parkinson’s disease-specific metabolic networks. Cereb Cortex 30:2867–2878. https://doi.org/10.1093/cercor/bhz280
Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028. https://doi.org/10.1038/ncomms5028
Schondorf DC, Ivanyuk D, Baden P, Sanchez-Martinez A, de Cicco S, Yu C, Giunta I, Schwarz LK, di Napoli G, Panagiotakopoulou V, Nestel S, Keatinge M, Pruszak J, Bandmann O, Heimrich B, Gasser T, Whitworth AJ, Deleidi M (2018) The NAD+ precursor nicotinamide riboside rescues mitochondrial defects and neuronal loss in iPSC and fly models of Parkinson’s disease. Cell Rep 23:2976–2988. https://doi.org/10.1016/j.celrep.2018.05.009
Schrag A, Horsfall L, Walters K, Noyce A, Petersen I (2015) Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol 14:57–64. https://doi.org/10.1016/S1474-4422(14)70287-X
Senkevich K, Gan-Or Z (2020) Autophagy lysosomal pathway dysfunction in Parkinson’s disease; evidence from human genetics. Parkinsonism Relat Disord 73:60–71. https://doi.org/10.1016/j.parkreldis.2019.11.015
Sidransky E (2004) Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab 83:6–15. https://doi.org/10.1016/j.ymgme.2004.08.015
Sidransky E (2012) Gaucher disease: insights from a rare Mendelian disorder. Discov Med 14:273–81
Sidransky E, Lopez G (2012) The link between the GBA gene and Parkinsonism. Lancet Neurol 11:986–998. https://doi.org/10.1016/S1474-4422(12)70190-4
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, de Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361:1651–1661. https://doi.org/10.1056/NEJMoa0901281
Simuni T, Uribe L, Cho HR, Caspell-Garcia C, Coffey CS, Siderowf A, Trojanowski JQ, Shaw LM, Seibyl J, Singleton A, Toga AW, Galasko D, Foroud T, Tosun D, Poston K, Weintraub D, Mollenhauer B, Tanner CM, Kieburtz K, Chahine LM, Reimer A, Hutten SJ, Bressman S, Marek K, Investigators P (2020) Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson’s progression markers initiative (PPMI): a cross-sectional study. Lancet Neurol 19:71–80. https://doi.org/10.1016/S1474-4422(19)30319-9
Smith L, Schapira AHV (2022) GBA variants and Parkinson disease: mechanisms and treatments. Cells. https://doi.org/10.3390/cells11081261
Srikanth MP, Jones JW, Kane M, Awad O, Park TS, Zambidis ET, Feldman RA (2021) Elevated glucosylsphingosine in Gaucher disease induced pluripotent stem cell neurons deregulates lysosomal compartment through mammalian target of rapamycin complex 1. Stem Cells Transl Med 10:1081–1094. https://doi.org/10.1002/sctm.20-0386
Stone DL, Carey WF, Christodoulou J, Sillence D, Nelson P, Callahan M, Tayebi N, Sidransky E (2000) Type 2 Gaucher disease: the collodion baby phenotype revisited. Arch Dis Child Fetal Neonatal Ed 82:F163–F166. https://doi.org/10.1136/fn.82.2.f163
Sucunza D, Rico AJ, Roda E, Collantes M, Gonzalez-Aseguinolaza G, Rodriguez-Perez AI, Penuelas I, Vazquez A, Labandeira-Garcia JL, Broccoli V, Lanciego JL (2021) Glucocerebrosidase gene therapy induces alpha-synuclein clearance and neuroprotection of midbrain dopaminergic neurons in mice and macaques. Int J Mol Sci. https://doi.org/10.3390/ijms22094825
Suzuki M, Fujikake N, Takeuchi T, Kohyama-Koganeya A, Nakajima K, Hirabayashi Y, Wada K, Nagai Y (2015a) Glucocerebrosidase deficiency accelerates the accumulation of proteinase K-resistant alpha-synuclein and aggravates neurodegeneration in a Drosophila model of Parkinson’s disease. Hum Mol Genet 24:6675–6686. https://doi.org/10.1093/hmg/ddv372
Suzuki T, Shimoda M, Ito K, Hanai S, Aizawa H, Kato T, Kawasaki K, Yamaguchi T, Ryoo HD, Goto-Inoue N, Setou M, Tsuji S, Ishida N (2015b) Correction: expression of human Gaucher disease gene GBA generates neurodevelopmental defects and ER stress in drosophila eye. PLoS ONE 10:e0135619. https://doi.org/10.1371/journal.pone.0135619
Swan M, Doan N, Ortega RA, Barrett M, Nichols W, Ozelius L, Soto-Valencia J, Boschung S, Deik A, Sarva H, Cabassa J, Johannes B, Raymond D, Marder K, Giladi N, Miravite J, Severt W, Sachdev R, Shanker V, Bressman S, Saunders-Pullman R (2016) Neuropsychiatric characteristics of GBA-associated Parkinson disease. J Neurol Sci 370:63–69. https://doi.org/10.1016/j.jns.2016.08.059
Szwedo AA, Dalen I, Pedersen KF, Camacho M, Backstrom D, Forsgren L, Tzoulis C, Winder-Rhodes S, Hudson G, Liu G, Scherzer CR, Lawson RA, Yarnall AJ, Williams-Gray CH, Macleod AD, Counsell CE, Tysnes OB, Alves G, Maple-Grodem J, Parkinson’s Incidence Cohorts, C. (2022) GBA and APOE impact cognitive decline in Parkinson’s disease: a 10-year population-based study. Mov Disord. https://doi.org/10.1002/mds.28932
Tayebi N, Walker J, Stubblefield B, Orvisky E, Lamarca ME, Wong K, Rosenbaum H, Schiffmann R, Bembi B, Sidransky E (2003) Gaucher disease with Parkinsonian manifestations: does glucocerebrosidase deficiency contribute to a vulnerability to parkinsonism? Mol Genet Metab 79:104–109. https://doi.org/10.1016/s1096-7192(03)00071-4
Valayannopoulos V (2013) Enzyme replacement therapy and substrate reduction therapy in lysosomal storage disorders with neurological expression. Handb Clin Neurol 113:1851–7. https://doi.org/10.1016/B978-0-444-59565-2.00055-1
Wang C, Cai Y, Gu Z, Ma J, Zheng Z, Tang BS, Xu Y, Zhou Y, Feng T, Wang T, Chen SD, Chan P, Chinese Parkinson Study, G (2014) Clinical profiles of Parkinson’s disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 35(725):e1-6. https://doi.org/10.1016/j.neurobiolaging.2013.08.012
Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, Duran R, Mencacci NE, Sawcer SJ, Barker RA (2013) Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain 136:392–399. https://doi.org/10.1093/brain/aws318
Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, Morrison A, Lwin A, Colegial C, Allman JM, Schiffmann R (2004) Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 82:192–207. https://doi.org/10.1016/j.ymgme.2004.04.011
Xu YH, Sun Y, Ran H, Quinn B, Witte D, Grabowski GA (2011) Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab 102:436–447. https://doi.org/10.1016/j.ymgme.2010.12.014
Yang H, Liu C, Zhong Y, Luo S, Monteiro MJ, Fang S (2010) Huntingtin interacts with the cue domain of gp78 and inhibits gp78 binding to ubiquitin and p97/VCP. PLoS ONE 5:e8905. https://doi.org/10.1371/journal.pone.0008905
Ysselstein D, Young TJ, Nguyen M, Padmanabhan S, Hirst WD, Dzamko N, Krainc D (2021) Evaluation of strategies for measuring lysosomal glucocerebrosidase activity. Mov Disord 36:2719–2730. https://doi.org/10.1002/mds.28815
Yun SP, Kim D, Kim S, Kim S, Karuppagounder SS, Kwon SH, Lee S, Kam TI, Lee S, Ham S, Park JH, Dawson VL, Dawson TM, Lee Y, Ko HS (2018) alpha-Synuclein accumulation and GBA deficiency due to L444P GBA mutation contributes to MPTP-induced parkinsonism. Mol Neurodegener 13:1. https://doi.org/10.1186/s13024-017-0233-5
Zhang Y, Sun QY, Zhao YW, Shu L, Guo JF, Xu Q, Yan XX, Tang BS (2015) Effect of GBA mutations on phenotype of Parkinson’s disease: a study on Chinese population and a meta-analysis. Parkinsons Dis 2015:916971. https://doi.org/10.1155/2015/916971
Zhang Y, Shu L, Sun Q, Zhou X, Pan H, Guo J, Tang B (2018a) Integrated genetic analysis of racial differences of common GBA variants in Parkinson’s disease: a meta-analysis. Front Mol Neurosci 11:43. https://doi.org/10.3389/fnmol.2018.00043
Zhang Y, Shu L, Zhou X, Pan H, Xu Q, Guo J, Tang B, Sun Q (2018b) A meta-analysis of GBA-related clinical symptoms in Parkinson’s disease. Parkinsons Dis 2018:3136415. https://doi.org/10.1155/2018/3136415
Ziegler SG, Eblan MJ, Gutti U, Hruska KS, Stubblefield BK, Goker-Alpan O, Lamarca ME, Sidransky E (2007) Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson disease. Mol Genet Metab 91:195–200. https://doi.org/10.1016/j.ymgme.2007.03.004
Zokaei N, McNeill A, Proukakis C, Beavan M, Jarman P, Korlipara P, Hughes D, Mehta A, Hu MT, Schapira AH, Husain M (2014) Visual short-term memory deficits associated with GBA mutation and Parkinson’s disease. Brain 137:2303–2311. https://doi.org/10.1093/brain/awu143
Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, Toker NJ, Jeon S, Fredriksen K, Mazzulli JR (2018) Reversible conformational conversion of alpha-synuclein into toxic assemblies by glucosylceramide. Neuron 97(92–107):e10. https://doi.org/10.1016/j.neuron.2017.12.012
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vieira, S.R.L., Schapira, A.H.V. Glucocerebrosidase mutations and Parkinson disease. J Neural Transm 129, 1105–1117 (2022). https://doi.org/10.1007/s00702-022-02531-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02531-3