Abstract
Risperidone is commonly used to treat different psychiatric disorders worldwide. Knowledge on dose–concentration relationships of risperidone treatment in children and adolescents with schizophrenia or other psychotic disorders is, however, scarce and no age-specific therapeutic ranges have been established yet. Multicenter data of a therapeutic drug monitoring service were analyzed to evaluate the relationship between risperidone dose and serum concentration of the active moiety (risperidone (RIS) plus its main metabolite 9-hydroxyrisperidone (9-OH-RIS)) in children and adolescents with psychotic disorders. Patient characteristics, doses, serum concentrations and therapeutic outcomes were assessed by standardized measures. The study also aimed to evaluate whether the therapeutic reference range for adults (20–60 ng/ml) is applicable for minors. In the 64 patients (aged 11–18 years) included, a positive correlation between daily dose and the active moiety (RISam) concentration was found (rs = 0.49, p = 0.001) with variation in dose explaining 24% (rs2 = 0.240) of the variability in serum concentrations. While the RISam concentration showed no difference, RIS as well 9-OH-RIS concentrations and the parent to metabolite ratio varied significantly in patients with co-medication of a CYP2D6 inhibitor. Patients with extrapyramidal symptoms (EPS) had on average higher RISam concentrations than patients without (p = 0.05). Considering EPS, the upper threshold of the therapeutic range of RISam was determined to be 33 ng/ml. A rough estimation method also indicated a possibly decreased lower limit of the preliminary therapeutic range in minors compared to adults. These preliminary data may contribute to the definition of a therapeutic window in children and adolescents with schizophrenic disorders treated with risperidone. TDM is recommended in this vulnerable population to prevent concentration-related adverse drug reactions.
Similar content being viewed by others
Introduction
Risperidone is commonly used worldwide to treat a variety of psychiatric symptoms and disorders in children and adolescents (Halfdanarson et al. 2017). Risperidone has FDA approval to treat schizophrenia in patients from 13 to 17 years as well as manic episodes in patients with bipolar disorder from 10 to 17 years. Furthermore, it is licensed in most countries for the short-term treatment of aggressive behavior in children and adolescents in the age of 5 years or older with mental retardation or irritability in children with autism spectrum disorder (ASD). However, in clinical practice, risperidone is often used off-label for a much broader range of mental disorders in young patients, such as tic disorders (Kim et al. 2005; Ghanizadeh and Haghighi 2014) or impulsive aggressive behavior associated with attention deficit hyperactivity disorder (Gadow et al. 2016).
While various randomized controlled trials and meta-analyses have confirmed the effectiveness/efficacy of risperidone (Lee et al. 2018) for different clinical indications, safety concerns remain given its adverse drug reactions (ADRs) profile. Especially weight gain and sedation seem to be more pronounced in youth (Safer 2004; Liu et al. 2019), but also extrapyramidal symptoms (EPS), hyperprolactinemia and impaired glucose tolerance are common ADRs that can lead to serious long-term health risks (Solmi et al. 2020). As some of these ADRs seem dose-dependent, optimal dose finding with the lowest effective dose is demanded. However, dosing in the age group of minors is difficult, due to age and developmental dependent differences in pharmacokinetics/pharmacodynamics (Egberts et al. 2011) and limited dosing evidence for off-label indications. Due to these benefit/risk concerns a close monitoring of the patient is important.
Therapeutic drug monitoring (TDM) provides a valid method for individual dose titration and careful monitoring and is strongly recommended by guidelines for adults treated with risperidone (Hiemke et al. 2018; Schoretsanitis et al. 2020). The parent component risperidone (RIS) is mainly metabolized by CYP2D6 into 9-hydroxyrisperidone (9-OH-RIS). The pharmacological characteristics and therapeutic effects of 9-OH-RIS are considered comparable to those of the parent compound (Heykants et al. 1994; Nazirizadeh et al. 2010). For this reason, TDM guidelines advise to determine serum concentrations of RIS and 9-OH-RIS and the sum of both, which is referred to as the active moiety (RISam) concentration in this paper. For adult patients with schizophrenia or bipolar disorder concentrations in the range of 20–60 ng/mL of the active moiety are recommended. Serum concentrations > 40 ng/mL should be targeted only in patients with insufficient or absence of therapeutic response to avoid neurological adverse reactions (Hiemke et al. 2018).
In children and adolescents, however, the relationships between risperidone dose and blood concentrations have not been clarified yet and age-specific recommended reference ranges of blood concentrations have not been defined for the different age or diagnostic groups. The few existing pharmacokinetic studies in pediatric patients all revealed linearity between daily dose and blood concentrations of the active moiety (Klampfl et al. 2010; Calarge and Miller del 2011; Fekete et al. 2021). In a population-based study, risperidone serum concentrations were comparable between children and adolescents and in line with results from adult patients (Thyssen et al. 2010). In a retrospective naturalistic study, however, lower dose-corrected serum concentrations of RISam were found in minors compared to adults possibly due to a higher renal clearance (Fekete et al. 2021). Other studies identified age and/or gender as well as body fat as potential modifying factors of risperidone concentrations (Aichhorn et al. 2007a; Calarge and Miller del 2011).
Several studies reported significant correlations between RIS or 9-OH-RIS concentrations and hyperprolactinemia (Troost et al. 2007; Duval et al. 2008; Migliardi et al. 2009; Roke et al. 2012; Ngamsamut et al. 2016) in youth, in contrast to another study that found no correlation between RISam concentrations and prolactin level increase in pediatric patients (Gagliano et al. 2004). A recent prospective cohort study in children and adolescents with ASD revealed that higher RISam trough concentrations predict higher prolactin levels, but also higher body mass index scores, more sedation and, interestingly, more effectiveness reducing irritability, indicating that a therapeutic window seems to exist for this indication (Kloosterboer et al. 2021). However, clinical studies assessing the relationships between psychotropic drug concentrations and clinical outcomes in children and adolescents are rare and characterized by methodological limitations (Kloosterboer et al. 2020).
The primary aim of this study was to assess the relationship between daily dose and serum concentration in children and adolescents treated with risperidone due to a schizophrenic psychosis using data from a routine TDM service. As a secondary aim, the influence of several patients’ and treatment characteristics on the serum concentrations was investigated. Furthermore, the aim was to explore whether the recommended range for blood concentrations of the active moiety in adults seems also valid and applicable for children and adolescents with schizophrenia.
Subjects and methods
Setting and study population
Patient data and blood samples were collected from three university hospital departments (Ulm, Wuerzburg, Vienna) and two departments of child and adolescent psychiatry (Ravensburg, Cologne Holweide) in Germany and Austria between 2006 and 2018 (Table 1). All participating hospitals are members of the competence network for TDM in child and adolescent psychiatry (www.tdm-kjp.com, Mehler-Wex et al. 2009) and used the routine TDM service of the service of the Center of Mental Health of the University Hospital Wuerzburg. Within this TDM service, serum concentrations of psychotropic drugs are measured and demographic, psychiatric and outcome data collected in a standardized way.
All patients—as part of routine patient care—received a physical–neurological and psychiatric examination, assessment of vital signs, body size, body weight, and laboratory analyses for hepatic and renal function. In addition, further patient characteristics (gender, age, diagnosis, comorbidity, nicotine use) and clinical data of drug treatment (e.g., dosage of risperidone, type and dosage of possible psychiatric co-medications) were determined. Furthermore, the date, reason for TDM analysis (e.g., ‘dose adjustment’ or ‘compliance control’) and the symptoms intended to treat with the medication (e.g., ‘positive symptoms’) were systematically assessed.
All patients up to the age of 18 years who were administered risperidone for the treatment of schizophrenic disorders (ICD-10 F2; mostly F20.x) or a drug induced psychotic disorder (ICD-10 F12.5, F19.5) independently of the setting of treatment (inpatient, outpatient, day-unit) were included. Patients were excluded from the study if steady-state conditions for blood taking were not fulfilled or relevant data were missing (e.g., daily dose, relevant patient information). Patients with drug induced psychotic disorder were excluded in case of ongoing consumption of illegal drugs.
The study was approved by the local ethic committee (study number 27/04) and carried out according to the Declaration of Helsinki. As the investigation of serum concentrations was part of the clinical routine of blood tests there was no necessity for written informed consent.
Measurement of risperidone serum concentrations
Analyses of RIS/ 9-OH-RIS serum concentrations were performed according to the consensus guidelines of TDM in Psychiatry of the AGNP (Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie; German society for neuropsychopharmacology and pharmacopsychiatry (Hiemke et al. 2018). In steady-state conditions, blood withdrawal from cubital veins was performed in 7.5 mL monovettes without anticoagulants and additives as trough value before the first daily intake of risperidone. The elimination half-life of RIS is 2–4 h, of its metabolite 9-OH-RIS 17–23 h, and of the active moiety 20 h (Mannens et al. 1993; Borison et al. 1994). Steady plasma concentrations are reached in poor metabolizers by days 5–6, in extensive metabolizers by day 1 (Chopko and Lindsley 2018). Date and time of blood withdrawal were noted. The blood was centrifuged at 1800 g for 10 min and analyzed immediately (samples from Wuerzburg) or within a few days after postage to the TDM laboratory in Wuerzburg.
Serum concentrations of RIS and 9-OH-RIS were analyzed by an automated column-switching method coupled to an isocratic high-performance liquid chromatography (HPLC) system and a variable ultraviolet detector as described in detail elsewhere (Klampfl et al. 2010). The intra-assay coefficients of variation determined from 10 analyses of both analytes (20 and 80 ng/mL) were in general less than 1%. The inter-assay variability for both analytes was in general less than 2%. The method was linear in a range of 4–200 ng/mL (RIS r2 = 0.99, 9-OH-RIS r2 = 0.99), and the lower limit of quantification was 3 ng/mL for both compounds. Chemicals and solvents with level of purity and RIS and 9-OH-RIS for calibration and controls were purchased commercially from Sigma-Aldrich, Munich, Germany. For patients with more than one concentration determination, the chronologically last available measurement was selected for this study.
Assessment of therapeutic outcomes
To assess the severity of psychopathology and the change of symptomatology, at the time of blood withdrawal, the Clinical Global Impression Scale was used (severity: CGI-S; improvement: CGI-I) and the change (improvement) therein (CGI-I) as a measure for effectiveness (Guy 1976). The following categories were applied adapting the CGI manual: 1 = (very) much better, 2 = moderately better, 3 = unchanged/ slightly worse, 4 = much worse, 0 = treatment effect not assessable. Patients with a CGI-I score of 1, 2 were defined as responders to drug treatment. The nature and severity of ADRs at the time period before blood taking were assessed using the Udvalg for Kliniske Undersogelser Side Effect Rating Scale (Lingjaerde et al. 1987) with the following categorization: 0 = no side effects; 1 = mild, 2 = moderate and 3 = severe side effects.
Data analysis
Statistical analyses were performed with the software SPSS, version 26. Means, medians and interquartile ranges (IQRs) were calculated for descriptive analyses. The Kolmogorov–Smirnoff test was used to evaluate variables for Gaussian distribution. The Spearman rank correlation coefficient (rs) was applied for not Gaussian distributed variables (serum concentrations), the Pearson coefficient (rp) for Gaussian distributed variables. Group differences were analyzed by independent t test and Mann–Whitney U test. Multiple linear regression analysis was used to determine influencing factors on RIS, 9-OH-RIS and RISam concentrations, e.g., sex, comedication, body weight, body mass index and cigarette smoking. A receiver-operating curve (ROC analysis) was performed to determine the upper limit of an age-specific therapeutic reference range, analyzing the RISam concentrations of patients without and with ADRs (EPS) to identify a cutoff value that separates patients with a high probability of such ADRs from those with a low probability. Statistical significance was defined as p ≤ 0.05. All values are presented as mean ± SD or as median and IQR whatever appropriate. The data set generated and analyzed during the current study is available from the corresponding author on reasonable request.
Results
Study population
The study population comprised 64 patients (70.3% male) with a mean (SD, range) age of 15.6 (1.7, 11–18) years, of whom three (4.7%) were younger than 13 years (Table 1). The vast majority (93.7%) had a diagnosis of a schizophrenic disorder (ICD-10 F2), 6.3% were diagnosed with psychotic symptoms in the context of consumption of illegal drugs (ICD-10 F1x.5). Almost 80% of the patients received one or more concomitant psychotropic medications, most commonly other antipsychotics. The severity of symptomatology was in most patients classified as ‘markedly ill’ (50.0%) or ‘severely ill’ (28.3%).
RIS/9-OH-RIS and active moiety concentrations in relation to risperidone doses and other covariates
The patients were treated with an average of 3.9 (SD 1.9, range 1–8) mg risperidone daily (Table 2). The mean body weight-adjusted dose was 0.02 mg/kg (SD 0.01, range 0.009–0.043). The daily dose did not differ in the subgroups classified according to gender (p = 0.46) or mode of pharmacotherapy (monotherapy versus co-medication) (p = 0.35). The mean dose-corrected RISam concentration (C/D) was 9.2 (ng/ml) / (mg/day) (SD 6.2, range 2.3–29.3). There was no statistically significant difference in the dose-corrected RISam concentrations between boys 8.2 (ng/ml)/(mg/d) and girls 10.6 (ng/ml)/(mg/day) (p = 0.24).
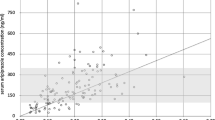
In 22 (34%) individuals, the RIS concentration was below, whereas the concentration of 9-OH-RIS was above the lower limit of quantification of the assay in all patients. The mean (SD) RISam concentration (n = 64) was 32.2 ng/ml (22.1). A large inter-patient variability of RISam concentrations was shown (IQR 17.0–43.8 ng/ml). Table 2 shows the measured serum concentrations in the total population and the different subsamples. In the whole sample a positive correlation between daily doses and RISam concentrations was found (rs = 0.49, p = 0.001), with the variation in dose explaining 24% (rs2 = 0.24) of the variability in serum concentrations (Fig. 1). Multiple linear regression analysis confirmed that sex (p = 0.79), body weight (p = 0.92), body mass index (p = 0.47) and the use of any psychotropic comedication versus monotherapy (0.07) had no significant influence on the RISam concentration. In contrast, cigarette consumption significantly influenced serum concentrations (p = 0.045) since RIS concentrations, 9-OH-RIS as well as RISam concentrations were each about 45% lower in smokers.
We identified six patients using a CYP2D6 inhibitor as concomitant medication, mainly fluoxetine (Table 2). Whereas there was no difference in the RISam concentration (p = 0.87), both the RIS (p = 0.004) and 9-OH-RIS levels (p = 0.026) as well as the RIS/9-OH-RIS ratio (p = 0.003) were significantly different between patients without and with a CYP2D6-inhibiting concomitant medication (more parent substance and less metabolite in patients using a CYP2D6 inhibitor).
Clinical positive and negative effects of risperidone treatment
Using CGI-I, 33.3% (n = 20) of the children and adolescents were rated as ‘(very) much better’ and 38.3% (n = 23) as ‘moderately better’. These 43 (71.6%) patients (were classified as responders. 26.7% of the patients were classified as non-responders as their state was rated as ‘unchanged/slightly worse’ (20%) or as ‘(much) worse’ (6.7%). Girls and boys were equally likely to be responders (p = 0.80). There was no significant difference of RISam concentrations in responders compared to non-responders (p = 0.77) (Table 2).
One or more ADRs were documented in the majority (63.5%) of the patients, with a non-significant higher frequency in girls (72.2%) than boys (60.0%) (p = 0.36). There also was no significant difference in the occurrence of ADRs in patients with and without concomitant psychotropic medication (p = 0.44). In the group of patients with ADRs and a rating of the treating child psychiatrist on severity (n = 32), 56.3% of ADRs were rated as ‘mild’, 43.7% as ‘moderate’. ‘Severe’ ADRs did not occur in any of the patients. Sedation/drowsiness (n = 18; 21.1% of all patients) and EPS (n = 13; 19.1% of all patients) were the most frequently reported ADRs. Other ADRs comprised an inner feeling of tension and agitation, hypersalivation, micturition and accommodation problems, gastrointestinal disturbances and cardiovascular disturbances. Prolactine was not measured in our study. Within a mean (SD) observation period from 156 ± 328 days (range 8–1190) in a small subgroup of 14 patients, an average weight gain (mean, SD) of 3.2 ± 4.6 kg (range: −1 to 14) was recorded. RISam concentrations did not differ in patients with and without any kind of ADRs (p = 0.60). However, patients with EPS (n = 13) had significantly higher (p = 0.05) mean RISam concentration (42.9 ng/ml) than patients without EPS (29.5 ng/ml), the same was true for RIS and 9-OH-RIS concentrations.
Determination of a preliminary therapeutic reference range of risperidone in children and adolescents with schizophrenic disorders
The comparison of RISam concentrations in our pediatric sample with the currently recommended therapeutic levels of the active moiety for adult patients with schizophrenia (20–60 ng/ml) revealed that 20 (31.3%) of all measured RISam concentrations were under the recommended therapeutic level for adults, and 8 (12.5%) were above. 36 (56.3%) of the RISam concentrations were within the therapeutic window—of which 25 (39.1%) were in the range between 20 and 40 ng/ml. No measured concentration reached the so-called laboratory alert level of 120 ng/ml, a threshold that obliges the laboratory to feedback immediately to the prescribing physician.
As patients with EPS had higher RISam concentrations than patients without EPS, we conducted a ROC analysis to identify the upper threshold of a possible age-specific therapeutic range. The area under the curve (AUC) was 0.63. The best distinction between sensitivity and specificity was found at a RISam concentration of 33 ng/ml indicating this cut off-value as the upper limit.
As long as valid data on a therapeutic reference range do not exist, a method described by the consensus guidelines for TDM in neuropsychopharmacology (Hiemke et al. 2018, for details see the discussion) can be applied for a rough estimation of a therapeutic window considering the range between the arithmetic mean ± one standard deviation of drug concentrations in the blood of responders to the drug therapy. We used this approach to estimate the lower limit of the therapeutic window of risperidone for children and adolescents with schizophrenic disorders. The mean (SD) RISam concentration of all responders was 31.9 (± 23.3) ng/ml leading to a suggested lower limit of about 9 (8.6) ng/ml.
Discussion
In this observational study in children and adolescents with schizophrenia, a positive linear relationship between daily dose and the RISam concentration was found. Smoking reduced RIS and 9-OH-RIS concentrations by about 45%. Concomitant medication of a CYP2D6 inhibitor had significant influence on the RIS concentrations (parent compound increased) as well as the metabolite 9-OH-RIS (reduced). Patients with EPS had higher RIS as well as 9-OH-RIS concentrations than patients without. More than a half of the pediatric patients had a RISam concentration within the recommended therapeutic range for adults with schizophrenia. Using an estimation method for the determination of a preliminary therapeutic window, our data point to a similar upper limit, but a decreased lower limit of a therapeutic range for children and adolescents with schizophrenia treated with risperidone.
Risperidone concentrations in relation to risperidone doses
In line with former studies on pharmacokinetics of risperidone in children and adolescents (Klampfl et al. 2010; Calarge and Miller del 2011; Fekete et al. 2021), a linear correlation between dose and blood concentration was observed. Our finding also is in line with a correlation between daily dose and risperidone blood concentrations seen in adult patients (He and Richardson 1995; Bowskill et al. 2012). As in previous pediatric TDM studies (Aman et al. 2007; Klampfl et al. 2010), and studies on adults (Aravagiri et al. 1998, 2003; Zhou et al. 2006; Castberg et al. 2017), concentrations in our pediatric sample were widely distributed and dosage accounted for only 24% of the variability in RISam serum concentrations. We found no significant influence of sex, body weight, body mass index on serum concentrations, neither an influence of age in our quite age-homogeneous sample (age 11–18 years). In the literature, an effect of old age on risperidone concentrations was observed with dose-adjusted concentrations about twice as high in the age of 80 compared to the age of 40 (Castberg et al. 2017). Heterogeneous results on sex differences were reported, as some studies found higher serum concentrations in females (e.g.,Aichhorn et al. 2007b; Castberg et al. 2017) and some in boys/males (Calarge and Miller del 2011). Others studies found no influence of sex (Thyssen et al. 2010; Pozzi et al. 2016; Kloosterboer et al. 2021), especially when the sample mainly consisted of boys like in our study, which is in line with the reported ratios of boys to girls of about 2.5:1 in schizophrenic patients with onset during childhood and adolescence (Russell et al. 1989). The differing results on sex might be confounded by age effects on renal clearance and maturation of the CYP system, duration of antipsychotic treatment as well as concomitant CYP2D6 inhibiting medication that was not controlled for in some studies. A higher renal clearance of 9-OH-RIS in minors might also be the reason for lower metabolite to parent-ratios (MPRs) of risperidone found in children/adolescents compared to adults (Fekete et al. 2021). In contrast to our results, other studies reported that a higher BMI correlates with higher 9-OH-RIS partly due a to altered CYP2D6 or CYP3A4 activity in obese patients (Paulzen et al. 2016).
In our sample cigarette consumption significantly influenced blood levels since RIS concentrations, 9-OH-RIS as well as RISam concentrations were each about 45% lower in smokers. The influence of cigarette smoking is vastly discussed in the literature with a well know effect of CYP1A2 induction by polycyclic aromatic hydrocarbons in cigarette smoke. Apart from the induction of CYP1A2 activity, Schoretsanitis et al. (2017) suggest from their retrospective TDM database study on nearly 700 patients, that smoking might exert an effect on additional CYP isoenzymes as well, most likely via CYP3A4. However, there is only one study which investigated the influence of polymorphisms in other cytochrome P450 systems than CYP2D6 for risperidone in children and adolescents, but did not find any significant effects of these genes (CYP3A4, CYP3A, P-glycoprotein) on risperidone pharmacokinetics (Kloosterboer et al. 2021).
While there was no difference in the RISam concentration under comedication with potential CYP2D6 inhibitors in our small subsample (n = 6), we identified that concomitant CYP2D6 inhibitors modified the MPR with an increased parent compound and reduced metabolite concentration. CYP2D6-inhibiting co-medications, have been demonstrated to increase the concentration–dose ratio of RIS, and coadministration of the potent inhibitor fluoxetine increased the RISam concentration by 50–75% (Chopko and Lindsley 2018); a finding in line with former results in adult patients (Berecz et al. 2002). In the literature a certain tissue distribution was found with a higher brain-to-plasma concentration ratio for RIS parent compound than for its active metabolite compared to other tissues, such as kidneys and liver (van Beijsterveldt et al. 1994; Aravagiri et al. 1998; Calarge and Miller del 2011). Therefore, co-administered CYP2D6 inhibitors during risperidone therapy could alter the serum concentrations of RIS and 9-OH-RIS and by the differing tissue distribution modify disposition in the brain, with a clinically significant implication on efficacy and tolerability. As clinical trials on polypharmacy to risperidone are scare, although polypharmacy is increasingly prevalent in daily clinical practice in youth (Safer et al. 2003; dosReis et al. 2005; Zito et al. 2008; Vloet et al. 2019), our real world data add to current knowledge.
Our data are only partially comparable with previous studies on risperidone blood levels in children and adolescents, as these studies have addressed patients with different diagnoses in mixed samples (Pozzi et al. 2016; Fekete et al. 2021) or other specific diagnostic subgroups, e.g., patients with behavioral disorders or ASD (Gagliano et al. 2004; Klampfl et al. 2010; Calarge and Miller del 2011; Kloosterboer et al. 2021). The mean daily risperidone doses, therefore, were comparably higher in our sample, consistent with the published literature to treat early onset schizophrenia (Armenteros and Davies 2006) and in contrast to the recommended lower dosages to treat behavioral problems (Ipser and Stein 2007).
Clinical outcome
Risperidone treatment was estimated as effective in most of the patients, as the symptoms of one third of the patients of the children and adolescents were rated as ‘(very) much better’ and more than 70.0% of the patients showed at least some benefit under the pharmacotherapy (‘moderately better’). ADRs were very common and documented for the majority of children and adolescents (63.5%). However, all ADRs were rated as ‘mild’ and ‘moderate’. ‘Severe’ ADRs did not occur in any of the patients. From the literature it is known, that ADRs correlate with RISam concentrations (Hiemke et al. 2018). In addition, elevated levels of dose-adjusted plasma 9-OH-RIS concentration were found to be associated with higher rates of ADR (Schoretsanitis et al. 2016). In line with literature in adult patients with schizophrenia (Spina et al. 2001), also in our sample, patients with EPS had significantly higher mean RIS, 9-OH-RIS and RISam concentrations than patients without. In the literature, in some studies a ratio > 1 of RIS/9-OH-RIS reflecting diminished CYP2D6 activity was associated with higher rates of ADR (Leon et al. 2007). In our sample, a subgroup of only 8 patients had an inverted ratio and no such correlation could be found.
Proposal of a preliminarily therapeutic range for children and adolescents
In all age groups, TDM of patients under risperidone treatment is recommended for personalized pharmacotherapy in clinical routine (for dose titration as well as special indications like assessment of medication adherence), as some ADRs correlate with drug concentrations and TDM increases the probability of response. For adults with schizophrenia, the therapeutic reference range of the RISam concentration is defined as 20–60 ng/ml according to results of PET studies and studies with therapeutically effective doses (Hiemke et al. 2018).
In children and adolescents treated with risperidone, no controlled TDM studies with fixed dose designs nor PET studies are available. A previous prospective clinical study indicates that a therapeutic window for risperidone in children and adolescents with ASD exists, as the RISam concentrations predicted both side effects and response (Kloosterboer et al. 2021). In addition, for children and adolescents treated with low doses of risperidone due to impulsive–aggressive behavior, a therapeutic window (8–26 ng/ml) was suggested (Klampfl et al. 2010). To our knowledge, valid data on a therapeutic reference range of risperidone in patients with (early onset) schizophrenia do not exist. A comparison of our data with the recommended therapeutic levels for adults revealed that more than half of the pediatric patients of our sample had a RISam concentration within the recommended therapeutic range for adults (56.3%) and 31.3% had a lower concentration.
Using a ROC analysis separating patients with EPS side effects from those without, an upper limit of a possible age-specific therapeutic range of RISam concentrations in children and adolescents with schizophrenic disorders of 33 ng/ml could be identified. Because of the limitations of our naturalistic study design it was not impossible to calculate the lower threshold level of the therapeutic range by a ROC analysis. Therefore, we referred to the method described in the consensus guidelines for TDM in neuropsychopharmacology (Hiemke et al. 2018) and used the arithmetic mean minus one standard deviation of the drug concentrations in the blood of responders to estimate the lower limit. Altogether, our findings suggest a therapeutic reference range of RISam from 9 to 33 ng/ml in children and adolescents, which is significantly lower than the range for adults (20–60 ng/ml) with schizophrenic disorders, but still higher than the range for children and adolescents treated with risperidone due to behavioral problems. Of course, this does not mean that all pediatric patients with schizophrenia already respond under lower RISam concentrations. However, dose has to be titrated very carefully, and we can underline for minors—like stated for adults in the TDM consensus guidelines for blood levels > 40 ng/mL (Hiemke et al. 2018)—that RISam > 33 ng/ml should be targeted only in cases of insufficient or absence of therapeutic response to avoid neurological adverse reactions. For the use of risperidone in other than the main indication, therapeutic concentration ranges have to be evaluated for the different age groups in further studies.
Limitations and strengths of the study
The findings of the present study must be interpreted in the context of several limitations. First, the influence of age and pubertal stage could not be determined, because only three children younger than 13 years were involved and the information on puberty was not recorded. Second, no genotyping was performed for the cytochrome P450 enzymes (CYP2D6, CYP3A4 and P-glycoprotein), that could influence pharmacokinetics. Third, the sample size was limited and especially the different subgroups of patients too small to identify variables with small effect sizes. Fourth, our study goes along with the typical limitations of an observational–naturalistic design including non-standardization of dose regimes and length of drug treatment before TDM assessment. Finally, concentration–effect relationships are very difficult to determine within naturalistic studies (Hiemke 2019) as placebo-responders and patients with side effects, who are likely to receive lower dosages, are not excluded from analysis as well as non-responders, who are likely to receive high dosages. To define a therapeutic window, further studies with more controlled study designs are necessary with higher case numbers and fixed dose regimens, fixed points of response assessment and the use of more specific clinical instruments to define clinical response for the different diagnostic entities or to investigate dose dependent side effects. Therefore, to complement naturalistic studies, there is the urgent need for more standardized investigations with larger sample sizes and controlled clinical designs. To learn more about the benefit–risk profile of psychotropic drug use in daily clinical practice a multicenter pharmacovigilance study (‘TDM-VIGIL’) was funded by the German Federal Institute for Drugs and Medical Devices (BfArM), Bonn, in collaboration with the ‘Competence Network on TDM in Child and Adolescent Psychiatry’’ (www.tdm-kjp.com). Using a modern internet-based patient registry, epidemiological and outcome data were assessed in a standardized way, including the results of standardized analysis of serum concentrations (Egberts et al. 2015, 2022).
As a strength, observational TDM studies like the present allow to gain data on dose–concentration relationships in ‘real world patients’, who are characterized by a variety of individual clinical characteristics and concomitant medications. For example, in clinical trials patients who use multiple psychotropic medications, are usually excluded. Only about 20% of the young patients in our sample were prescribed risperidone monotherapy, all others received at least one concomitant psychotropic drug in addition to risperidone, although monotherapy with antipsychotics is recommended as the first-line treatment for schizophrenia in most clinical guidelines (e.g.,Lehman et al. 2004; Miller et al. 2004 German Association for Psychiatry, Psychotherapy and Psychosomatics 2019), while polypharmacy with psychotropic agents in the treatment of schizophrenia is common in clinical practice (Hashimoto et al. 2021). Nearly half of the patients received two or more antipsychotics (antipsychotic polypharmacy), which is common in the treatment of schizophrenic disorders for the management of refractory psychotic symptoms (Correll and Gallego 2012). The observed considerable inter-individual differences in serum concentrations could partly be influenced by 2D6 activity either by genetic variation or by the use of 2D6 inhibiting comedication. There are more than 70 variants identified for CYP2D6 (Zhou 2009a, b) and an effect of CYP2D6 variants on the clearance of RIS metabolites is proven: poor metabolizers achieved RISam concentrations up to 3.3-fold (intermediate 1.6-fold) higher at the same doses compared to extensive metabolizers (Riedel et al. 2005; Locatelli et al. 2010; Hendset et al. 2014). In the future, genotyping of CYP2D6 as part of the clinical routine—additionally to TDM—might help for a more personalized pharmacotherapy with individual dose optimization.
Conclusion
Our study described the distribution of risperidone serum concentrations of pediatric patients with schizophrenia in a real life-setting, significantly increasing the amount of available data in this vulnerable population. A significant correlation between daily dose and RISam concentration was found with a high inter individual variability. As patients with EPS had higher risperidone concentrations than patients without, TDM is recommended to prevent ADRs. Our data hint on a lower therapeutic concentration range in children and adolescents compared to the therapeutic window established for adults. Considering individual pharmacokinetic parameters, TDM provides an effective pharmacovigilance tool in the pediatric population.
References
Aichhorn W, Marksteiner J, Walch T, Zernig G, Hinterhuber H, Stuppaeck C, Kemmler G (2007a) Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. J Child Adolesc Psychopharmacol 17:665–674
Aichhorn W, Whitworth AB, Weiss EM, Hinterhuber H, Marksteiner J (2007b) Differences between men and women in side effects of second-generation antipsychotics. Nervenarzt 78:45–52
Aman MG, Vinks AA, Remmerie B, Mannaert E, Ramadan Y, Masty J, Lindsay RL, Malone K (2007) Plasma pharmacokinetic characteristics of risperidone and their relationship to saliva concentrations in children with psychiatric or neurodevelopmental disorders. Clin Ther 29:1476–1486
Aravagiri M, Marder SR, Nuechterlein KH, Gitlin MJ (2003) Intra- and interindividual variations in steady-state plasma concentrations of risperidone and 9-hydroxyrisperidone in schizophrenic patients treated chronically with various doses of risperidone. Ther Drug Monit 25:657–664
Aravagiri M, Marder SR, Wirshing D, Wirshing WC (1998) Plasma concentrations of risperidone and its 9-hydroxy metabolite and their relationship to dose in schizophrenic patients: simultaneous determination by a high performance liquid chromatography with electrochemical detection. Pharmacopsychiatry 31:102–109
Armenteros JL, Davies M (2006) Antipsychotics in early onset Schizophrenia: Systematic review and meta-analysis. Eur Child Adolesc Psychiatry 15:141–148
Berecz R, LLerena A, de la Rubia A, Gomez J, Kellermann M, Dorado P, Degrell I (2002) Relationship between risperidone and 9-hydroxy-risperidone plasma concentrations and CYP2D6 enzyme activity in psychiatric patients. Pharmacopsychiatry 35:231–234
Borison RL, Diamond B, Pathiraja A, Meibach RC (1994) Pharmacokinetics of risperidone in chronic schizophrenic patients. Psychopharmacol Bull 30:193–197
Bowskill SV, Handley SA, Fisher DS, Flanagan RJ, Patel MX (2012) Risperidone and total 9-hydroxyrisperidone in relation to prescribed dose and other factors: data from a therapeutic drug monitoring service, 2002–2010. Ther Drug Monit 34:349–355
Calarge CA, del Miller D (2011) Predictors of risperidone and 9-hydroxyrisperidone serum concentration in children and adolescents. J Child Adolesc Psychopharmacol 21:163–169
Castberg I, Westin AA, Skogvoll E, Spigset O (2017) Effects of age and gender on the serum levels of clozapine, olanzapine, risperidone, and quetiapine. Acta Psychiatr Scand 136:455–464
Chopko TC, Lindsley CW (2018) Classics in chemical neuroscience: risperidone. ACS Chem Neurosci 9:1520–1529
Correll CU, Gallego JA (2012) Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am 35:661–681
dosReis S, Zito JM, Safer DJ, Gardner JF, Puccia KB, Owens PL (2005) Multiple psychotropic medication use for youths: a two-state comparison. J Child Adolesc Psychopharmacol 15:68–77
Duval F, Guillon MS, Mokrani MC, Crocq MA, Garcia Duarte F (2008) Relationship between prolactin secretion, and plasma risperidone and 9-hydroxyrisperidone concentrations in adolescents with schizophreniform disorder. Psychoneuroendocrinology 33:255–259
Egberts KM, Mehler-Wex C, Gerlach M (2011) Therapeutic drug monitoring in child and adolescent psychiatry. Pharmacopsychiatry 44:249–253
Egberts K, Karwautz A, Plener PL, Mehler-Wex C, Kölch M, Dang SY, Taurines R, Romanos M, Gerlach M (2015) Pharmacovigilance in child and adolescent psychiatry. Z Kinder Jugendpsychiatr Psychother 43:21–28
Egberts KM, Gelach M, Correll C, Plener PL, Malzahn U, Heuschmann P, Unterecker S, Scherf-Clavel M, Rock H, Antony G, Briegel W, Fleichhaker C, Häge A, Hellenschmidt T, Imgart H, Kaess M, Karwautz A, Kölch M, Reitzle K, Renner T, Reuter-Dang S-Y, Rexroth C, Schulte-Körne G, Theisen F, Walitza S, Wewetzer C, Fekete S, Taurines R, Romanos M (2022) Serious adverse drug reactions in children and adolescents treated on- and off-label with antidepressants and antipsychotics in clinical practice. Pharmacopsychiatry. https://doi.org/10.1055/a-1716-1856
Fekete S, Scherf-Clavel M, Gerlach M, Romanos M, Kittel-Schneider S, Unterecker S, Egberts K (2021) Dose-corrected serum concentrations and metabolite to parent compound ratios of venlafaxine and risperidone from childhood to old age. Pharmacopsychiatry 54:117–125
Gadow KD, Brown NV, Arnold LE, Buchan-Page KA, Bukstein OG, Butter E, Farmer CA, Findling RL, Kolko DJ, Molina BS, Rice RR Jr, Schneider J, Aman MG (2016) Severely aggressive children receiving stimulant medication versus stimulant and risperidone: 12-month follow-up of the TOSCA Trial. J Am Acad Child Adolesc Psychiatry 55:469–478
Gagliano A, Germano E, Pustorino G, Impallomeni C, D’Arrigo C, Calamoneri F, Spina E (2004) Risperidone treatment of children with autistic disorder: effectiveness, tolerability, and pharmacokinetic implications. J Child Adolesc Psychopharmacol 14:39–47
Ghanizadeh A, Haghighi A (2014) Aripiprazole versus risperidone for treating children and adolescents with tic disorder: a randomized double blind clinical trial. Child Psychiatry Hum Dev 45:596–603
Guy W (1976) ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare Publication, Rockville
Halfdanarson O, Zoega H, Aagaard L, Bernardo M, Brandt L, Fuste AC, Furu K, Garuoliene K, Hoffmann F, Huybrechts KF, Kalverdijk LJ, Kawakami K, Kieler H, Kinoshita T, Litchfield M, Lopez SC, Machado-Alba JE, Machado-Duque ME, Mahesri M, Nishtala PS, Pearson SA, Reutfors J, Saastamoinen LK, Sato I, Schuiling-Veninga CCM, Shyu YC, Skurtveit S, Verdoux H, Wang LJ, Yahni CZ, Bachmann CJ (2017) International trends in antipsychotic use: A study in 16 countries, 2005–2014. Eur Neuropsychopharmacol 27:1064–1076
Hashimoto N, Yasui-Furukori N, Hasegawa N, Ishikawa S, Numata S, Hori H, Iida H, Ichihashi K, Furihata R, Murata A, Tsuboi T, Takeshima M, Kyou Y, Komatsu H, Kubota C, Ochi S, Takaesu Y, Usami M, Nagasawa T, Hishimoto A, Miura K, Matsumoto J, Ohi K, Yamada H, Inada K, Watanabe K, Shimoda K, Hashimoto R (2021) Characteristics of discharge prescriptions for patients with schizophrenia or major depressive disorder: real-world evidence from the Effectiveness of Guidelines for Dissemination and Education (EGUIDE) psychiatric treatment project. Asian J Psychiatr 63:102744
He H, Richardson JS (1995) A pharmacological, pharmacokinetic and clinical overview of risperidone, a new antipsychotic that blocks serotonin 5-HT2 and dopamine D2 receptors. Int Clin Psychopharmacol 10:19–30
Hendset M, Molden E, Knape M, Hermann M (2014) Serum concentrations of risperidone and aripiprazole in subgroups encoding CYP2D6 intermediate metabolizer phenotype. Ther Drug Monit 36:80–85
Heykants J, Huang ML, Mannens G, Meuldermans W, Snoeck E, Van Beijsterveldt L, Van Peer A, Woestenborghs R (1994) The pharmacokinetics of risperidone in humans: a summary. J Clin Psychiatry 55(Suppl):13–17
Hiemke C (2019) Concentration-effect relationships of psychoactive drugs and the problem to calculate therapeutic reference ranges. Ther Drug Monit 41:174–179
Hiemke C, Bergemann N, Clement HW, Conca A, Deckert J, Domschke K, Eckermann G, Egberts K, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Hefner G, Helmer R, Janssen G, Jaquenoud E, Laux G, Messer T, Mossner R, Muller MJ, Paulzen M, Pfuhlmann B, Riederer P, Saria A, Schoppek B, Schoretsanitis G, Schwarz M, Gracia MS, Stegmann B, Steimer W, Stingl JC, Uhr M, Ulrich S, Unterecker S, Waschgler R, Zernig G, Zurek G, Baumann P (2018) Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51:9–62
Ipser J, Stein DJ (2007) Systematic review of pharmacotherapy of disruptive behavior disorders in children and adolescents. Psychopharmacology 191:127–140
Kim BN, Lee CB, Hwang JW, Shin MS, Cho SC (2005) Effectiveness and safety of risperidone for children and adolescents with chronic tic or Tourette disorders in Korea. J Child Adolesc Psychopharmacol 15:318–324
Klampfl K, Taurines R, Preuss A, Burger R, Rothenhofer S, Wewetzer C, Pfuhlmann B, Fegert J, Gerlach M, Mehler-Wex C (2010) Serum concentrations, therapeutic response and side effects in children and adolescents with impulsive-aggressive symptoms during risperidone therapy. Pharmacopsychiatry 43:58–65
Kloosterboer SM, de Winter BCM, Reichart CG, Kouijzer MEJ, de Kroon MMJ, van Daalen E, Ester WA, Rieken R, Dieleman GC, van Altena D, Bartelds B, van Schaik RHN, Nasserinejad K, Hillegers MHJ, van Gelder T, Dierckx B, Koch BCP (2021) Risperidone plasma concentrations are associated with side effects and effectiveness in children and adolescents with autism spectrum disorder. Br J Clin Pharmacol 87:1069–1081
Kloosterboer SM, Vierhout D, Stojanova J, Egberts KM, Gerlach M, Dieleman GC, Hillegers MHJ, Passe KM, Gelder TV, Dierckx B, Koch BCP (2020) Psychotropic drug concentrations and clinical outcomes in children and adolescents: a systematic review. Expert Opin Drug Saf 19:873–890
Lee ES, Vidal C, Findling RL (2018) A focused review on the treatment of pediatric patients with atypical antipsychotics. J Child Adolesc Psychopharmacol 28:582–605
Lehman AF, Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Goldberg R, Green-Paden LD, Tenhula WN, Boerescu D, Tek C, Sandson N, Steinwachs DM (2004) The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull 30:193–217
Leon J, Susce MT, Pan RM, Wedlund PJ, Orrego ML, Diaz FJ (2007) A study of genetic (CYP2D6 and ABCB1) and environmental (drug inhibitors and inducers) variables that may influence plasma risperidone levels. Pharmacopsychiatry 40:93–102
Lingjaerde O, Ahlfors UG, Bech P et al (1987) The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100
Liu XI, Schuette P, Burckart GJ, Green DJ, La J, Burnham JM, Rakhmanina N, Robb A, Huang SM, van den Anker JN (2019) A comparison of Pediatric and Adult Safety Studies for Antipsychotic and Antidepressant Drugs submitted to the United States Food and Drug Administration. J Pediatr 208:236–242
Locatelli I, Kastelic M, Koprivsek J, Kores-Plesnicar B, Mrhar A, Dolzan V, Grabnar I (2010) A population pharmacokinetic evaluation of the influence of CYP2D6 genotype on risperidone metabolism in patients with acute episode of schizophrenia. Eur J Pharm Sci 41:289–298
Mannens G, Huang ML, Meuldermans W, Hendrickx J, Woestenborghs R, Heykants J (1993) Absorption, metabolism, and excretion of risperidone in humans. Drug Metab Dispos 21:1134–1141
Mehler-Wex C, Kolch M, Kirchheiner J, Antony G, Fegert JM, Gerlach M (2009) Drug monitoring in child and adolescent psychiatry for improved efficacy and safety of psychopharmacotherapy. Child Adolesc Psychiatry Ment Health 3:14
Migliardi G, Spina E, D’Arrigo C, Gagliano A, Germano E, Siracusano R, Diaz FJ, de Leon J (2009) Short- and long-term effects on prolactin of risperidone and olanzapine treatments in children and adolescents. Prog Neuropsychopharmacol Biol Psychiatry 33:1496–1501
Miller A, Hall CS, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Ereshefsky L, Essock SM, Finnerty M, Marder SR, Miller DD, McEvoy JP, Rush AJ, Saeed SA, Schooler NR, Shon SP, Stroup S, Tarin-Godoy B (2004) The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2003 update. J Clin Psychiatry 65:500–508
Nazirizadeh Y, Vogel F, Bader W, Haen E, Pfuhlmann B, Grunder G, Paulzen M, Schwarz M, Zernig G, Hiemke C (2010) Serum concentrations of paliperidone versus risperidone and clinical effects. Eur J Clin Pharmacol 66:797–803
Ngamsamut N, Hongkaew Y, Vanwong N, Srisawasdi P, Puangpetch A, Chamkrachangpada B, Tan-Khum T, Limsila P, Sukasem C (2016) 9-Hydroxyrisperidone-induced hyperprolactinaemia in Thai children and adolescents with autism spectrum disorder. Basic Clin Pharmacol Toxicol 119:267–272
Paulzen M, Haen E, Stegmann B, Hiemke C, Grunder G, Lammertz SE, Schoretsanitis G (2016) Body mass index (BMI) but not body weight is associated with changes in the metabolism of risperidone; A pharmacokinetics-based hypothesis. Psychoneuroendocrinology 73:9–15
Pozzi M, Cattaneo D, Baldelli S, Fucile S, Capuano A, Bravaccio C, Sportiello L, Bertella S, Auricchio F, Bernardini R, Ferrajolo C, Guastella G, Mani E, Carnovale C, Pisano S, Rafaniello C, Riccio MP, Rizzo R, Scuderi MG, Sperandeo S, Villa L, Pascotto A, Molteni M, Rossi F, Radice S, Clementi E (2016) Therapeutic drug monitoring of second-generation antipsychotics in pediatric patients: an observational study in real-life settings. Eur J Clin Pharmacol 72:285–293
Riedel M, Schwarz MJ, Strassnig M, Spellmann I, Muller-Arends A, Weber K, Zach J, Muller N, Moller HJ (2005) Risperidone plasma levels, clinical response and side-effects. Eur Arch Psychiatry Clin Neurosci 255:261–268
Roke Y, Buitelaar JK, Boot AM, Tenback D, van Harten PN (2012) Risk of hyperprolactinemia and sexual side effects in males 10–20 years old diagnosed with autism spectrum disorders or disruptive behavior disorder and treated with risperidone. J Child Adolesc Psychopharmacol 22:432–439
Russell AT, Bott L, Sammons C (1989) The phenomenology of schizophrenia occurring in childhood. J Am Acad Child Adolesc Psychiatry 28:399–407
Safer DJ (2004) A comparison of risperidone-induced weight gain across the age span. J Clin Psychopharmacol 24:429–436
Safer DJ, Zito JM, DosReis S (2003) Concomitant psychotropic medication for youths. Am J Psychiatry 160:438–449
Schoretsanitis G, Haen E, Grunder G, Stegmann B, Schruers KR, Hiemke C, Lammertz SE, Paulzen M (2016) Pharmacokinetic drug-drug interactions of mood stabilizers and risperidone in patients under combined treatment. J Clin Psychopharmacol 36:554–561
Schoretsanitis G, Haen E, Stegmann B, Hiemke C, Grunder G, Paulzen M (2017) Effect of smoking on risperidone pharmacokinetics - a multifactorial approach to better predict the influence on drug metabolism. Schizophr Res 185:51–57
Schoretsanitis G, Kane JM, Correll CU, Marder SR, Citrome L, Newcomer JW, Robinson DG, Goff DC, Kelly DL, Freudenreich O, Piacentino D, Paulzen M, Conca A, Zernig G, Haen E, Baumann P, Hiemke C, Gründer G, American Society of Clinical Psychopharmacology, Pharmakopsychiatrie (2020) Blood Levels to Optimize Antipsychotic Treatment in Clinical Practice: A Joint Consensus Statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry 19:19cs13169
Solmi M, Fornaro M, Ostinelli EG, Zangani C, Croatto G, Monaco F, Krinitski D, Fusar-Poli P, Correll CU (2020) Safety of 80 antidepressants, antipsychotics, anti-attention-deficit/hyperactivity medications and mood stabilizers in children and adolescents with psychiatric disorders: a large scale systematic meta-review of 78 adverse effects. World Psychiatry 19:214–232
Spina E, Avenoso A, Facciola G, Salemi M, Scordo MG, Ancione M, Madia AG, Perucca E (2001) Relationship between plasma risperidone and 9-hydroxyrisperidone concentrations and clinical response in patients with schizophrenia. Psychopharmacology 153:238–243
Thyssen A, Vermeulen A, Fuseau E, Fabre MA, Mannaert E (2010) Population pharmacokinetics of oral risperidone in children, adolescents and adults with psychiatric disorders. Clin Pharmacokinet 49:465–478
Troost PW, Lahuis BE, Hermans MH, Buitelaar JK, van Engeland H, Scahill L, Minderaa RB, Hoekstra PJ (2007) Prolactin release in children treated with risperidone: impact and role of CYP2D6 metabolism. J Clin Psychopharmacol 27:52–57
van Beijsterveldt LE, Geerts RJ, Leysen JE, Megens AA, Van den Eynde HM, Meuldermans WE, Heykants JJ (1994) Regional brain distribution of risperidone and its active metabolite 9-hydroxy-risperidone in the rat. Psychopharmacology 114:53–62
Vloet TD, Egberts K, Taurines R, Wewetzer C, Mehler-Wex C, Plener PL, Romanos M, Gerlach M (2019) Polypharmacy of psychotropic drugs in child and adolescent psychiatry in Germany - rather the rule than the exception. Z Kinder Jugendpsychiatr Psychother 47:193–202
Zhou SF (2009a) Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet 48:689–723
Zhou SF (2009b) Polymorphism of human cytochrome P450 2D6 and its clinical significance: part II. Clin Pharmacokinet 48:761–804
Zhou ZL, Li X, Peng HY, Yu XY, Yang M, Su FL, Wang F, Zhu RH, Deng CY, Lin QX, Wang CY, Li WB, Lin SG, Li HD (2006) Multiple dose pharmacokinetics of risperidone and 9-hydroxyrisperidone in Chinese female patients with schizophrenia. Acta Pharmacol Sin 27:381–386
Zito JM, Safer DJ, Sai D, Gardner JF, Thomas D, Coombes P, Dubowski M, Mendez-Lewis M (2008) Psychotropic medication patterns among youth in foster care. Pediatrics 121:e157-163
Acknowledgements
We gratefully acknowledge the laboratory of TDM of the Center of Mental Health of the University Hospital Wuerzburg, Prof. Juergen Deckert and staff. We thank Prof. Toine Egberts, University Medical Centre Utrecht, for his help with the data analysis and reflection on previous versions of the manuscript. The participating hospitals are members in the ‘Competence Network on TDM in Child and Adolescent Psychiatry’, which was supported by the German Federal Ministry of Education and Research (BMBF-FKZ: 01EZ0937) as well as by the ‘Verein zur Durchführung neurowissenschaftlicher Tagungen e.V.’ Paulsborner-Strasse 44, 14193 Berlin. The mentioned multicenter pharmacovigilance study (‘TDM-VIGIL’) is established in cooperation with this network and funded by the German Federal Institute for Drugs and Medical Devices (BfArM), Bonn (BfArM-Reference Number: 73.05/3832-397285/12).
Funding
Open Access funding enabled and organized by Projekt DEAL. We gratefully acknowledge the laboratory of TDM of the Center of Mental Health of the University Hospital Wuerzburg, Prof. Juergen Deckert and staff. We thank Prof. Toine Egberts, University Medical Centre Utrecht, for his help with the data analysis and reflection on previous versions of the manuscript. The participating hospitals are members in the ‘Competence Network on TDM in Child and Adolescent Psychiatry’, which was supported by the German Federal Ministry of Education and Research (BMBF-FKZ: 01EZ0937) as well as by the ‘Verein zur Durchführung neurowissenschaftlicher Tagungen e.V.’ Paulsborner-Strasse 44, 14193 Berlin. The mentioned multicenter pharmacovigilance study (‘TDM-VIGIL’) is established in cooperation with this network and funded by the German Federal Institute for Drugs and Medical Devices (BfArM), Bonn (BfArM-Reference Number: 73.05/3832-397285/12).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taurines, R., Fekete, S., Preuss-Wiedenhoff, A. et al. Therapeutic drug monitoring in children and adolescents with schizophrenia and other psychotic disorders using risperidone. J Neural Transm 129, 689–701 (2022). https://doi.org/10.1007/s00702-022-02485-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-022-02485-6