Abstract
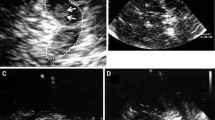
In Huntington’s disease (HD), a neurodegenerative-inherited disease, chorea as the typical kind of movement disorder is described. Beside chorea, however, all other kinds of movement disturbances, such as bradykinesia, dystonia, tremor or myoclonus can occur. Aim of the current study was to investigate alterations in the echogenicity of basal ganglia structures in different Huntington’s disease phenotypes. 47 patients with manifest and genetically confirmed HD were recruited. All participants underwent a thorough neurological examination. According to a previously described method, classification into predominantly choreatic, mixed or bradykinetic-rigid motor phenotypes was performed depending on subscores of the Unified Huntington’s Disease Rating Scale. In addition, findings in juvenile HD were compared to adult HD. Transcranial sonography was performed by investigators blinded to clinical classification. There were no significant differences in basal ganglia echogenicities between the three phenotypes. Size of echogenic area of substantia nigra (SN) correlated positively with CAG repeat and bradykinesia subscore, and negatively with age of onset and chorea subscore. Comparing juvenile and adult HD subtypes, SN hyperechogenicity was significantly more often detectable in the juvenile form (100 vs. 29.3 %, p = 0.002). Regarding echogenicity of caudate or lentiform nuclei, no significant differences were detected. HD patients with the juvenile variant exhibit marked hyperechogenicity of substantia nigra. No significant differences in basal ganglia echogenicities between predominantly choreatic, mixed or bradykinetic-rigid motor phenotypes were detected.


Similar content being viewed by others

References
Becker G, Berg D (2001) Neuroimaging in basal ganglia disorders: perspectives for transcranial ultrasound. Mov Disord 16(1):23–32
Becker G, Naumann M, Scheubeck M, Hofmann E, Deimling M, Lindner A, Gahn G, Reiners C, Toyka KV, Reiners K (1997) Comparison of transcranial sonography, magnetic resonance imaging, and single photon emission computed tomography findings in idiopathic spasmodic torticollis. Mov Disord 12(1):79–88
Berardelli A, Noth J, Thompson PD, Bollen EL, Curra A, Deuschl G, van Dijk JG, Topper R, Schwarz M, Roos RA (1999) Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord 14(3):398–403
Berg D (2011) Substantia nigra hyperechogenicity is a risk marker of Parkinson’s disease: yes. J Neural Transm 118(4):613–619
Berg D, Bandmann O (2013) Biomarkers for PD: how can we approach complexity? Neurology 80(7):608–609
Berg D, Siefker C, Ruprecht-Dorfler P, Becker G (2001) Relationship of substantia nigra echogenicity and motor function in elderly subjects. Neurology 56(1):13–17
Berg D, Merz B, Reiners K, Naumann M, Becker G (2005) Five-year follow-up study of hyperechogenicity of the substantia nigra in Parkinson’s disease. Mov Disord 20(3):383–385
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7(11):1044–1055
Berg D, Behnke S, Seppi K, Godau J, Lerche S, Mahlknecht P, Liepelt-Scarfone I, Pausch C, Schneider N, Gaenslen A, Brockmann K, Srulijes K, Huber H, Wurster I, Stockner H, Kiechl S, Willeit J, Gasperi A, Fassbender K, Gasser T, Poewe W (2013a) Enlarged hyperechogenic substantia nigra as a risk marker for Parkinson’s disease. Mov Disord 28(2):216–219
Berg D, Godau J, Seppi K, Behnke S, Liepelt-Scarfone I, Lerche S, Stockner H, Gaenslen A, Mahlknecht P, Huber H, Srulijes K, Klenk J, Fassbender K, Maetzler W, Poewe W (2013b) The PRIPS study: screening battery for subjects at risk for Parkinson’s disease. Eur J Neurol 20(1):102–108
Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464(7288):529–535
Bruggemann N, Hagenah J, Stanley K, Klein C, Wang C, Raymond D, Ozelius L, Bressman S, Saunders-Pullman R (2011) Substantia nigra hyperechogenicity with LRRK2 G2019S mutations. Mov Disord 26(5):885–888
Gaenslen A (2010) Transcranial sonography in dystonia. Int Rev Neurobiol 90:179–187
Hagenah J, Konig IR, Sperner J, Wessel L, Seidel G, Condefer K, Saunders-Pullman R, Klein C, Bruggemann N (2010) Life-long increase of substantia nigra hyperechogenicity in transcranial sonography. Neuroimage 51(1):28–32
Hagenah J, Konig IR, Kotter C, Seidel G, Klein C, Bruggemann N (2011) Basal ganglia hyperechogenicity does not distinguish between patients with primary dystonia and healthy individuals. J Neurol 258(4):590–595
Hart EP, Marinus J, Burgunder JM, Bentivoglio AR, Craufurd D, Reilmann R, Saft C, Roos RA (2013) Better global and cognitive functioning in choreatic versus hypokinetic-rigid Huntington’s disease. Mov Disord 28(8):1142–1145
Huntington Study Group (1996) Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov Disord 11(2):136–142
Krogias C, Eyding J, Postert T (2010) Transcranial sonography in Huntington’s disease. Int Rev Neurobiol 90:237–257
Krogias C, Strassburger K, Eyding J, Gold R, Norra C, Juckel G, Saft C, Ninphius D (2011) Depression in patients with Huntington disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J Psychiatry Neurosci 36(3):187–194
Muller M, Leavitt BR (2014) Iron dysregulation in Huntington’s disease. J Neurochem 130(3):328–350
Nance MA, Myers RH (2001) Juvenile onset Huntington’s disease–clinical and research perspectives. Ment Retard Dev Disabil Res Rev 7(3):153–157
Naumann M, Becker G, Toyka KV, Supprian T, Reiners K (1996) Lenticular nucleus lesion in idiopathic dystonia detected by transcranial sonography. Neurology 47(5):1284–1290
Oliva D, Carella F, Savoiardo M, Strada L, Giovannini P, Testa D, Filippini G, Caraceni T, Girotti F (1993) Clinical and magnetic resonance features of the classic and akinetic-rigid variants of Huntington’s disease. Arch Neurol 50(1):17–19
Penney JB Jr, Vonsattel JP, MacDonald ME, Gusella JF, Myers RH (1997) CAG repeat number governs the development rate of pathology in Huntington’s disease. Ann Neurol 41(5):689–692
Postert T, Lack B, Kuhn W, Jergas M, Andrich J, Braun B, Przuntek H, Sprengelmeyer R, Agelink M, Buttner T (1999) Basal ganglia alterations and brain atrophy in Huntington’s disease depicted by transcranial real time sonography. J Neurol Neurosurg Psychiatry 67(4):457–462
Quarrell O, O’Donovan KL, Bandmann O, Strong M (2012) The prevalence of juvenile Huntington’s disease: a review of the literature and meta-analysis. PLoS Curr 4:e4f8606b742ef3
Quintanilla RA, Johnson GV (2009) Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res Bull 80(4–5):242–247
Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS (2011) Pathophysiology of Huntington’s disease: time-dependent alterations in synaptic and receptor function. Neuroscience 198:252–273
Reiner A, Shelby E, Wang H, Demarch Z, Deng Y, Guley NH, Hogg V, Roxburgh R, Tippett LJ, Waldvogel HJ, Faull RL (2013) Striatal parvalbuminergic neurons are lost in Huntington’s disease: implications for dystonia. Mov Disord 28(12):1691–1699
Roos RA (2010) Huntington’s disease: a clinical review. Orphanet J Rare Dis 5(1):40
Rudzinska M, Krawczyk M, Wojcik-Pedziwiatr M, Szczudlik A, Tomaszewski T (2013) Tremor in neurodegenerative ataxias, Huntington disease and tic disorder. Neurol Neurochir Pol 47(3):232–240
Saft C, Zange J, Andrich J, Muller K, Lindenberg K, Landwehrmeyer B, Vorgerd M, Kraus PH, Przuntek H, Schols L (2005) Mitochondrial impairment in patients and asymptomatic mutation carriers of Huntington’s disease. Mov Disord 20(6):674–679
Saft C, Lauter T, Kraus PH, Przuntek H, Andrich JE (2006) Dose-dependent improvement of myoclonic hyperkinesia due to valproic acid in eight Huntington’s disease patients: a case series. BMC Neurology 6:11
Sanchez-Pernaute R, Kunig G, del Alba Barrio A, de Yebenes JG, Vontobel P, Leenders KL (2000) Bradykinesia in early Huntington’s disease. Neurology 54(1):119–125
Sierra M, Sanchez-Juan P, Martinez-Rodriguez MI, Gonzalez-Aramburu I, Garcia-Gorostiaga I, Quirce MR, Palacio E, Carril JM, Berciano J, Combarros O, Infante J (2013) Olfaction and imaging biomarkers in premotor LRRK2 G2019S-associated Parkinson disease. Neurology 80(7):621–626
Stuwe SH, Goetze O, Lukas C, Klotz P, Hoffmann R, Banasch M, Orth M, Schmidt WE, Gold R, Saft C (2013) Hepatic mitochondrial dysfunction in manifest and premanifest Huntington disease. Neurology 80(8):743–746
Thompson PD, Berardelli A, Rothwell JC, Day BL, Dick JP, Benecke R, Marsden CD (1988) The coexistence of bradykinesia and chorea in Huntington’s disease and its implications for theories of basal ganglia control of movement. Brain 111(Pt 2):223–244
Todd G, Taylor JL, Baumann D, Butler JE, Duma SR, Hayes M, Carew-Jones F, Piguet O, Behnke S, Ridding MC, Berg D, Double KL (2010) Substantia nigra echomorphology and motor cortex excitability. Neuroimage 50(4):1351–1356
van den Bogaard SJ, Dumas EM, Roos RA (2013) The role of iron imaging in Huntington’s disease. Int Rev Neurobiol 110:241–250
Vonsattel JP, Keller C, Cortes Ramirez EP (2011) Huntington’s disease—neuropathology. Handb Clin Neurolo 100:83–100
Walker FO (2007) Huntington’s disease. Lancet 369(9557):218–228
Walter U (2011) Substantia nigra hyperechogenicity is a risk marker of Parkinson’s disease: no. J Neural Transm 118(4):607–612
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60(1):74–77
Walter U, Krolikowski K, Tarnacka B, Benecke R, Czlonkowska A, Dressler D (2005) Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology 64(10):1726–1732
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, Berg D (2007) Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol 33(1):15–25
Walter U, Kanowski M, Kaufmann J, Grossmann A, Benecke R, Niehaus L (2008) Contemporary ultrasound systems allow high-resolution transcranial imaging of small echogenic deep intracranial structures similarly as MRI: a phantom study. Neuroimage 40(2):551–558
Walter U, Buttkus F, Benecke R, Grossmann A, Dressler D, Altenmuller E (2012) Sonographic alteration of lenticular nucleus in focal task-specific dystonia of musicians. Neuro-degenerative diseases 9(2):99–103
Acknowledgments
The authors would like to thank all participants for their time and interest in this study.
Full financial disclosure
R. Hoffmann, Dr. K. Strassburger-Krogias and Dr. S. Meves have no competing interests. Dr. C. Saft received honorarium from Temmler Pharma GmbH & Co.KG and Desitin Arzneimittel GmbH for scientific talks, compensation in the context of the Registry Study of the Euro-HD-Network, in the context of the MitoNet-study, the ACR16-Study (Neurosearch), the AFQ-Study (Novartis), the Selisistat-Studies (Siena Biotech) and received research support for a research project with Teva Pharma GmbH, Biogen and the ‘Cure Huntington’s Disease Initiative´ (CHDI). Dr. T. Lücke received research support for research projects from Merck-Serono, Teva Pharma, Vitafo, Actelion Pharmaceutical LTD, Merz, Meta X and Labmed GmbH. He received honorarium for scientific talks or activities in scientific expert rounds from Actelion LTD and Merck Serono. Dr. G. Ellrichmann received speakers or scientific grant support from BiogenIdec, TEVA Pharma and Novartis Pharma. Dr. C. Krogias received travel grants for scientific meetings from Bayer Vital and Bristol-Myers Squibb.
Financial disclosure related to research covered in this article
The authors have nothing to declare.
Funding
No funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saft, C., Hoffmann, R., Strassburger-Krogias, K. et al. Echogenicity of basal ganglia structures in different Huntington’s disease phenotypes. J Neural Transm 122, 825–833 (2015). https://doi.org/10.1007/s00702-014-1335-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-014-1335-7