Abstract
Purpose
Bevacizumab’s use in recurrent high-grade glioma is controversial. This study evaluates outcomes in recurrent high-grade glioma patients receiving bevacizumab alone or combined with chemotherapy as a late-line treatment.
Methods
We retrospectively analyzed patients treated with bevacizumab alone or combined with chemotherapy for high-grade gliomas who showed tumor progression after multiple treatment attempts. Overall survival (OS) and progression-free survival (PFS) were analyzed with Kaplan–Meier curves. Predictors of PFS according to prognostic variables were assessed with regression analysis.
Results
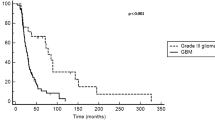
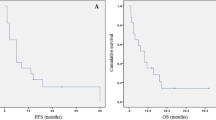
Between 2010 and 2022, 31 consecutive patients received bevacizumab alone or combined with chemotherapy as a late-line treatment for recurrent high-grade gliomas. Of these patients, 14 (45.2%) were responders according to RANO criteria, and 17 (54.8%) showed progressive or stable disease. OS at 3, 6, and 12 months was 80.3%, 62.1%, and 43.5. PFS was 48.4%, 34.3%, and 21.8%, respectively. In the multivariate survival analysis, the only factor independently associated with PFS was smaller 2D tumor size in post-contrast T1-weighted MRI at bevacizumab initiation (p = 0.02). Median time-to-progression was 3 months (95%CI: 1–4) in the unmethylated MGMT promoter group and 6 (95%CI: 1–11) in the methylated MGMT promoter group. This difference was not statistically significant (p = 0.37).
Conclusions
Bevacizumab alone or in combination with chemotherapy could be beneficial as a late-line therapy in a subset of patients with recurrent high-grade glioma. Small 2D tumor size in post-contrast T1 weighted MRI at bevacizumab initiation was independently associated with prolonged time to progression.


Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- TMZ:
-
Temozolomide
- VEGF:
-
Vascular endothelial growth factor
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- MRI:
-
Magnetic resonance imaging
- 11C-MET-PET:
-
Positron emission tomography imaging with 11C methionine
- KPS:
-
Karnofsky Performance Scale
- RANO:
-
Response Assessment in Neuro-Oncology
- IQR:
-
Interquartile ranges
- IDH:
-
Isocitrate dehydrogenases
- EGFR:
-
Epidermal growth factor receptor
- MGMT:
-
O6 methylguanine-DNA methyl-transferase
References
Chen C, Huang R, MacLean A, Muzikansky A, Mukundan S, Wen PY, Norden AD (2013) Recurrent high-grade glioma treated with bevacizumab: prognostic value of MGMT methylation, EGFR status and pretreatment MRI in determining response and survival. J Neurooncol 115(2):267–276
Chinot OL, Wick W, Mason W et al (2014) Bevacizumab plus radiotherapy–temozolomide for newly diagnosed glioblastoma. N Engl J Med 370(8):709–722
Detti B, Scoccianti S, Teriaca MA, Maragna V, Lorenzetti V, Lucidi S, Bellini C, Greto D, Desideri I, Livi L (2021) Bevacizumab in recurrent high-grade glioma: a single institution retrospective analysis on 92 patients. Radiol med 126(9):1249–1254
Erdem-Eraslan L, van den Bent MJ, Hoogstrate Y et al (2016) Identification of patients with recurrent glioblastoma who may benefit from combined bevacizumab and CCNU therapy: a report from the BELOB trial. Cancer Res 76(3):525–534
Field KM, Simes J, Nowak AK et al (2015) Randomized phase 2 study of carboplatin and bevacizumab in recurrent glioblastoma. Neuro Oncol 17(11):1504–1513
Gilbert MR, Dignam JJ, Armstrong TS et al (2014) A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(8):699–708
Herrlinger U, Tzaridis T, Mack F et al (2019) Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA–09): a randomised, open-label, phase 3 trial. The Lancet 393(10172):678–688
Hughes KL, O’Neal CM, Andrews BJ, Westrup AM, Battiste JD, Glenn CA (2021) A systematic review of the utility of amino acid PET in assessing treatment response to bevacizumab in recurrent high-grade glioma. Neuro-Oncol Adv 3(1):vdab003
Hutterer M, Nowosielski M, Putzer D et al (2011) O -(2–18 F-fluoroethyl)-L-tyrosine PET predicts failure of antiangiogenic treatment in patients with recurrent high-grade glioma. J Nucl Med 52(6):856–864
Kim MM, Umemura Y, Leung D (2018) Bevacizumab and glioblastoma: past, present, and future directions. Cancer J 24(4):180–186
Lefranc F, Le Rhun E, Kiss R, Weller M (2018) Glioblastoma quo vadis : will migration and invasiveness reemerge as therapeutic targets? Cancer Treat Rev 68:145–154
Liu LY, Ji MS, Nguyen NT et al (2019) Patterns of long-term survivorship following bevacizumab treatment for recurrent glioma: a case series. CNS Oncology 8(2):CN35
Modh A, Bergman D, Schultz L, Snyder J, Mikkelsen T, Ryu S, Siddiqui MS, Walbert T (2018) RTHP-06. Randomized prospective trial of stereotactic radiosurgery versus chemotherapy for recurrent malignant glioma after second-line chemotherapy. Neuro-Oncol 20(suppl_6):vi226–vi226
Morisse MC, Etienne-Selloum N, Bello-Roufai D et al (2019) Long-term survival in patients with recurrent glioblastoma treated with bevacizumab: a multicentric retrospective study. J Neurooncol 144(2):419–426
Robles Irizarry L, Hambardzumyan D, Nakano I, Gladson CL, Ahluwalia MS (2012) Therapeutic targeting of VEGF in the treatment of glioblastoma. Expert Opin Ther Targets 16(10):973–984
Seyedmirzaei H, Shobeiri P, Turgut M, Hanaei S, Rezaei N (2021) VEGF levels in patients with glioma: a systematic review and meta-analysis. Rev Neurosci 32(2):191–202
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10(5):459–466
Stupp R, Wong ET, Kanner AA et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48(14):2192–2202
Stupp R, Taillibert S, Kanner A et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318(23):2306
Taal W, Oosterkamp HM, Walenkamp AME et al (2014) Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol 15(9):943–953
Tabouret E, Barrie M, Thiebaut A, Matta M, Boucard C, Autran D, Loundou A, Chinot O (2013) Limited impact of prognostic factors in patients with recurrent glioblastoma multiforme treated with a bevacizumab-based regimen. J Neurooncol 114(2):191–198
Weller M, van den Bent M, Preusser M et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18(3):170–186
Wick W, Fricke H, Junge K et al (2014) A phase II, randomized, study of weekly APG101 + reirradiation versus reirradiation in progressive glioblastoma. Clin Cancer Res 20(24):6304–6313
Wick W, Gorlia T, Bendszus M et al (2017) Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med 377(20):1954–1963
Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S (2011) Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 9(4):403–407
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Mehdi Yahia-Cherif. Mehdi Yahia-Cherif wrote the first draft, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study obtained institutional ethics committee approval by the Ethics committee ULB-Erasme, number: 2022/204.
Consent to participate
This retrospective study was approved by the institutional ethics committee approval, and patients’ consents were waived.
Consent for publication
This retrospective study was approved by the institutional ethics committee approval, and patients’ consents were waived.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tumor - Glioma.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yahia-Cherif, M., Luce, S., De Witte, O. et al. Late-line treatment with bevacizumab alone or in combination with chemotherapy in recurrent high-grade gliomas. Acta Neurochir 165, 693–699 (2023). https://doi.org/10.1007/s00701-023-05524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05524-7