Abstract
Background
Besides 5-aminolevulinic acid (5-ALA), liver enzyme elevation after brain tumor surgery can be caused by anesthesia and medications. In this retrospective study, we determined whether preoperative 5-ALA administration is associated with postoperative elevation of liver enzymes (PELE) in brain tumor patients and identified predictive factors for PELE in patients treated with 5-ALA.
Methods
In 179 patients undergoing brain tumor surgery with preoperative normal values of liver enzymes, laboratory data on serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and total bilirubin (T.bil) levels were collected preoperatively and through postoperative day (POD) 45.
Results
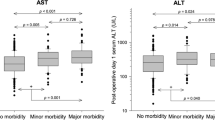
Ninety-nine PELEs (ALT, 56; AST, 34; ALP, 5; and TB, 4) were observed in 62 (34.6%) patients. Four (4.2%) patients treated with 5-ALA showed grade 3 elevation of transaminases based on the Common Terminology Criteria for Adverse Effects. Preoperative 5-ALA treatment was predictive of PELE (odds ratio [95% confidence interval], 2.30 [1.14–4.67]; P = 0.021). In patients treated with 5-ALA (n = 95), 70 PELEs (ALT, 39; AST, 22; ALP, 5; and TB, 4) were observed in 41 (43.2%) patients and significant predictive factors for PELE were preoperative ALT level (1.10 [1.04–1.17]; P = 0.001) and body mass index (BMI, 1.29 [1.08–1.56]; P = 0.006). In patients treated with 5-ALA, 13 and 36 patients, of 39 patients whose maximum postoperative ALT levels > 40 U/L, showed the normal value of serum ALT on PODs 14 and 45, respectively. Only three patients showed ALT elevation > 40 U/L on PODs 15–45, with a downward trend.
Conclusions
The use of 5-ALA for brain tumor surgery in patients with preoperative normal values of liver enzymes was associated with increased transient PELE, but a low incidence of severely elevated liver transaminases levels. When 5-ALA is administered to patients with the upper normal value of preoperative serum ALT and overweight, attention is paid to PELE.

Similar content being viewed by others
References
Björnsson E (2008) Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand 118:281–290
Björnsson ES (2017) Drug-induced liver injury due to antibiotics. Scand J Gastroenterol 52:617–623
Chen TL, Ueng TH, Chen SH, Lee PH, Fan SZ, Liu CC (1995) Human cytochrome P450 mono-oxygenase system is suppressed by propofol. Br J Anaesth 74:558–562
Chung IWH, Eljamel S (2013) Risk factors for developing oral 5-aminolevulenic acid-induced side effects in patients undergoing fluorescence guided resection. Photodiagn Photodyn Ther 10:362–367
Cowan RE, Jackson BT, Grainger SL, Thompson RP (1991) Effects of anesthetic agents and abdominal surgery on liver blood flow. Hepatology 14:1161–1166
Giannini EG, Testa R, Savarino V (2005) Liver enzyme alteration: a guide for clinicians. CMAJ 172:367–379
Honorato-Cia C, Martinez-Simón A, Cacho-Asenjo E, Guillén-Grima F, Tejada-Solís S, Diez-Valle R (2015) Safety profile of 5-aminolevulinic acid as a surgical adjunct in clinical practice: a review of 207 cases from 2008 to 2013. J Neurosurg Anesthesiol 27:304–309
Kim J, Jo I (2010) Relationship between body mass index and alanine aminotransferase concentration in non-diabetic Korean adults. Eur J Clin Nutr 64:169–175
Lee WM (2003) Drug-induced hepatotoxicity. N Engl J Med 349:474–485
Lichtenbelt BJ, Olofsen E, Dahan A, van Kleef JW, Struys MMRF, Vuyk J (2010) Propofol reduces the distribution and clearance of midazolam. Anesth Analg 110:1597–1606
Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi A-R, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36:E10
Offersen CM, Skjoeth-Rasmussen J (2017) Evaluation of the risk of liver damage from the use of 5-aminolevulinic acid for intra-operative identification and resection in patients with malignant gliomas. Acta Neurochir 159:145–150
Rahimzadeh P, Safari S, Faiz SHR, Alavian SM (2014) Anesthesia for patients with liver disease. Hepat Mon 14:e19881
Rai R, Nagral S, Nagral A (2012) Surgery in a patient with liver disease. J Clin Exp Hepatol 2:238–246
Rinella ME, Alonso E, Rao S, Whitington P, Fryer J, Abecassis M, Superina R, Flamm SL, Blei AT (2001) Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl 7:409–414
Ruland S, Aiyagari V (2007) Cerebral autoregulation and blood pressure lowering. Hypertension 49:977–978
Sachar M, Anderson KE, Ma X (2016) Protoporphyrin IX: the good, the bad, and the ugly. J Pharmacol Exp Ther 356:267–275
Savarese D (2018) Common terminology criteria for adverse events - UpToDate. https://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events. Accessed 09 Mar 2018
Stepp H, Stummer W (2018) 5-ALA in the management of malignant glioma. Lasers Surg Med 50:399–419
Stummer W, Goetz C (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J, Group A-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff R-O, Groups EOfRaToCBTaRO, Group NCIoCCT (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Teixidor P, Arráez MÁ, Villalba G, Garcia R, Tardáguila M, González JJ, Rimbau J, Vidal X, Montané E (2016) Safety and efficacy of 5-aminolevulinic acid for high grade glioma in usual clinical practice: a prospective cohort study. PLoS One 11:e0149244
Webber J, Kessel D, Fromm D (1997) Side effects and photosensitization of human tissues after aminolevulinic acid. J Surg Res 68:31–37
Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C, Chinot O, Ram Z, Reifenberger G, Soffietti R, Wick W, Glioma EAfN-OETFoM (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403
Woods CP, Hazlehurst JM, Tomlinson JW (2015) Glucocorticoids and non-alcoholic fatty liver disease. J Steroid Biochem Mol Biol 154:94–103
Funding
Seoul National University Bundang Hospital provided financial support in the form of academic funding (grant number: 02-2014-033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
The sponsor had no role in the design or conduct of this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This retrospective study was conducted after approval from the Institutional Review Board of Seoul National University Hospital (IRB no. 1811-078-985). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (name of institute/committee) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Because this was a retrospective study, for this type of study formal consent is not required.
Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Comments
A study that once more demonstrated the safety of using 5-ALA as adjunct for malignant brain tumor surgery with regards to the medical influence of the substance. Still the most dangerous is to have the tumor and transient elevation of liver enzymes are of less relevance.
Jane Skjoth-Rasmussen
Copenhagen, Denmark
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presentation at a conference
None
This article is part of the Topical Collection on Brain Tumors
Rights and permissions
About this article
Cite this article
Kim, JH., Yoon, HK., Lee, HC. et al. Preoperative 5-aminolevulinic acid administration for brain tumor surgery is associated with an increase in postoperative liver enzymes: a retrospective cohort study. Acta Neurochir 161, 2289–2298 (2019). https://doi.org/10.1007/s00701-019-04053-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-04053-6