Abstract
Apiaceae tribe Scandiceae includes species with diverse fruits that depending upon their morphology are dispersed by gravity, carried away by wind, or transported attached to animal fur or feathers. This diversity is particularly evident in Scandiceae subtribe Daucinae, a group encompassing species with wings or spines developing on fruit secondary ribs. In this paper, we explore fruit evolution in 86 representatives of Scandiceae and outgroups to assess adaptive shifts related to the evolutionary switch between anemochory and epizoochory and to identify possible dispersal syndromes, i.e., patterns of covariation of morphological and life-history traits that are associated with a particular vector. We also assess the phylogenetic signal in fruit traits. Principal component analysis of 16 quantitative fruit characters and of plant height did not clearly separate species having different dispersal strategies as estimated based on fruit appendages. Only presumed anemochory was weakly associated with plant height and the flattening of mericarps with their accompanying anatomical changes. We conclude that in Scandiceae, there are no distinct dispersal syndromes, but a continuum of fruit morphologies relying on different dispersal vectors. Phylogenetic mapping of ten discrete fruit characters on trees inferred by nrDNA ITS and cpDNA sequence data revealed that all are homoplastic and of limited use for the delimitation of genera. Spines evolved from wings developing on secondary ribs. We hypothesize that spines cannot form on primary ribs because these contain vascular bundles that may constrain such a transformation. We describe a new subtribe for Artedia and propose three new combinations in Daucus.
Similar content being viewed by others
Introduction
Plant dispersal syndromes describe patterns of covariation of morphological, anatomical and life-history traits that are associated with a particular vector of diaspore dispersal (e.g., wind, water, animals) and its mode (e.g., endozoochory vs. epizoochory). Such patterns may serve as simple phenotypic predictors of dispersal given that the correct set of phenotypic traits explaining a large fraction of variation in effective dispersal is identified and such syndromes are extrapolated to other systems and conditions. This, however, is problematic when patterns of covariation vary themselves continuously across environmental conditions (Ronce and Clobert 2012).
Adaptive shifts toward a particular dispersal vector can affect the entire fruit morphology and anatomy. For example, the selection for lighter diaspores may constrain not only seed size but also endosperm defense structures like a sclerified seed coat. If so, dispersal syndromes in fruit multidimensional morphospace are represented by separate clouds of species points. Alternatively, the adaptation to different vectors can affect only a limited number of traits, such as those immediately related to dispersal like fruit appendages (wings, hooks, etc.). If so, the dispersal mode is a poor predictor of fruit morphology and, consequently, its placement in the multidimensional morphospace. To test these hypotheses, we examine fruit evolution in the umbellifer tribe Scandiceae subtribe Daucinae and outgroups. This subtribe is particularly useful for such analyses because our previous study identified several evolutionary switches between anemochory and epizoochory in this group (Banasiak et al. 2016).
In Apiaceae, the fruits are dry schizocarps and the dispersal units are single-seeded mericarps. These diaspores are usually described based on their shape and appendages as anemochorous (dispersed by wind), epizoochorous (carried away on animal fur or feathers), and hydrochorous (floating on water). Fruits without any distinct adaptations to dispersal are regarded as barochorous (gravity dispersed) or autochorous (self-dispersing). Fleshy and thus endozoochorous fruits occur only in Apiopetalum, Mackinlaya, and Azorella sect. Stilbocarpa (Grushvitsky et al. 1969).
Anemochorous fruits involve a set of characters including dorsal or, rarely, lateral compression of the mericarps, with wing-like projections of various origins. These fruit types occur in at least 80 genera of umbellifers (Liu et al. 2006). Wind-dispersed fruits are particularly common in tribes Selineae including Angelica, Peucedanum, and Lomatium (Spalik et al. 2004), Scandiceae encompassing Ferula (Kurzyna-Młynik et al. 2008), Thapsia (Weitzel et al. 2014), and Laserpitium (Downie et al. 2010; Lee and Downie 1999), and Tordylieae comprising Tordylium, Heracleum, and the African ‘peucedanoid’ genera (Winter et al. 2008; Yu et al. 2011). The only study on wind dispersal in winged-fruited umbellifers concluded that its effectiveness as a mode of dispersal is quite low (Jongejans and Telenius 2001).
Adaptations to external animal dispersal (epizoochory) in Apiaceae have not been yet systematically reviewed. Bristles and hooks increase attachment and retention potential on animal fur (Römermann et al. 2005; Tackenberg et al. 2006) and promote gene flow among populations (Williams 1994; Williams and Guries 1994). Stiff bristles occur in several genera of Scandiceae subtribe Scandicinae (Spalik et al. 2001). In several species of Oenanthe, Seseli, Magydaris, and Hohenackeria, calyx teeth are extant in fruits and bristle-like (Arenas Posada and García Martín 1993). Spiny fruits developing from secondary ribs occur in former members of tribe Caucalideae; such a development was considered unique among umbellifers and therefore synapomorphic (Heywood 1982; Heywood and Dakshini 1971). However, the results of molecular analyses not only separated these spiny-fruited taxa into two clades, namely Scandiceae subtribes Torilidinae and Daucinae (Lee and Downie 1999, 2000; Lee et al. 2001), but also demonstrated that Daucinae are closely related to winged-fruited species formerly placed in tribe Laserpitieae (Downie et al. 2000a). Maximum parsimony mapping of secondary ribs characters onto a molecular phylogenetic tree of Daucinae revealed 14 character state changes and at least seven switches between winged and spiny ribs suggesting considerable evolutionary lability of fruit dispersal syndromes in this clade of umbellifers (Banasiak et al. 2016).
When analyzing dispersal syndromes in Apiaceae, it is important to note that fruit appendages are not necessarily the best indicators of dispersal strategy, because fruit morphology and anatomy are also subject to selection pressures other than dispersal. For epizoochory, mericarp mass, width, and length are also important predictors of its attachment and retention potential on animal fur (Römermann et al. 2005; Tackenberg et al. 2006). Therefore, umbellifer fruits may represent a full spectrum of adaptations to diverse dispersing agents rather than distinct dispersal syndromes. If dispersal syndromes do constitute sets of covarying characters, then a change from winged to spiny fruits or the opposite should invoke respective changes in other morphological characters. If there is no morphological covariation, then there are no true, discrete syndromes but a continuum of fruit morphologies in the adaptive landscape. The major aim of this paper is to check whether umbellifer fruits exhibit distinct dispersal syndromes or whether the adaptations to wind or animal dispersal are superficial and do not affect fruit morphology and anatomy. We examine the evolution of fruit morphology and anatomy of tribe Scandiceae in the context of presumed dispersal syndromes, with particular attention to former members of tribes Caucalideae and Laserpitieae—now segregated between subtribes Daucinae and Torilidinae—that represent considerable variation in secondary ribs appendages. We only include representative samples of subtribes Scandicinae and Ferulinae. The former was subject of our earlier studies of fruit evolution (Piwczyński et al. 2015; Spalik et al. 2001) while the latter includes only anemochorous species (Korovin 1947) and therefore does not represent any notable variation in dispersal strategy.
Apart from selection pressure, fruit morphology and anatomy may also reflect a common evolutionary history, i.e., fruit similarity is synapomorphic rather than resulting from convergence. The phylogenetic significance of fruit characters for spiny-fruited taxa placed in the former tribe Caucalideae was evaluated previously by Lee et al. (2001), but their study included only a small sample of winged-fruited taxa. Therefore, a secondary aim of this paper is to assess the phylogenetic signal in fruit morphology and, particularly, to confirm whether the genera of Daucinae that were recently redefined based solely on molecular data (Banasiak et al. 2016) are also supported with fruit characters.
Materials and methods
Species selection and morphological data
The selection of taxa was based on earlier phylogenetic studies of tribe Scandiceae (Downie et al. 2000a) and its subtribe Daucinae (Banasiak et al. 2016) and the availability of ripe fruits for morphological analyses. Sampled taxa represented a wide array of fruit morphologies occurring in this group. Effectively, 86 species and subspecies were examined for molecular and morphological characters. These included representatives of Scandiceae subtribes Daucinae (39 taxa sampled of approximately 90 in total), Torilidinae (14 of about 20), Scandicinae (22 of about 120), Ferulinae (7 of about 170), and Artedia squamata that does not have a clear subtribal placement. Outgroup taxa included representatives of tribes Smyrnieae (two species) and Aciphylleae (one species).
The external characters of mature fruits were examined using a Nikon SMZ-U dissecting microscope (8–160× magnification). For anatomical studies, the fruits were soaked in water and hand-cut; dissections were stained with phloroglucinol or safranin–fast green and were examined using a Nikon Optiphot 2 optical microscope (40–1000× magnification). Drawings were made using a drawing tube or based on photographs taken using a Nikon Microflex HFX-DX photograph system. Sources of all material examined are provided in Online Resource 1.
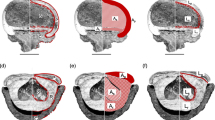
Because our aim was not only to assess dispersal syndromes but also to delimit newly redefined genera, we included a variety of fruit morphological and anatomical characters. Some of these characters like fruit size and compression are related to dispersal mode. Other features may result from different selection pressures; for instance, various forms of fruit sclerification or vittae filled with resin may serve as endosperm protection against herbivory. Sixteen quantitative (Nos. 1–16) and ten qualitative (Nos. 18–27, Table 1 and Online Resource 2) fruit characters were considered. We also included approximate plant height (No. 17) and life history (No. 29) because they may affect optimal dispersal mode (Cohen and Plitmann 1997; Harper et al. 1970). Additionally, character No. 28 describes the mode of fruit dispersal as estimated based on fruit appendages. Morphological (external) quantitative characters were scored based on a sample of 3–10 fully ripe fruits (depending on their availability on herbarium specimens), while anatomical characters were usually based on a single dissected fruit (see Fig. 1 for details of measurements). Approximate plant height and life history were obtained from various floristic accounts.
Mericarp anatomy and characters considered in this study. See Table 1 for character and character state descriptions
Molecular data
Four molecular markers were considered: nuclear ribosomal DNA internal transcribed spacer (nrDNA ITS), plastid rpoB-trnC intergenic spacer, and introns in plastid rps16 and rpoC1 genes. These regions were used in previous studies of Apiaceae phylogeny (Downie et al. 2000b, 2010; Lee and Downie 2000), including Daucinae (Banasiak et al. 2016). Seventy-two sequences were obtained for this study (Online Resource 1).
The laboratory procedures, including PCR and sequencing reactions, followed earlier studies (Banasiak et al. 2016; Panahi et al. 2015). Total genomic DNA was isolated from ca. 20 mg of dried leaf tissue using a DNeasy Plant Mini Kit (Qiagen, Hilden, Germany). For nrDNA ITS, the DNA samples were PCR-amplified using primers N-nc18S10 and C26A (Wen and Zimmer 1996). For some difficult accessions, the ITS 1 and ITS 2 regions were amplified separately using the following pairs of primers: 18S-ITS1-F and 5.8S-ITS1-R for ITS 1, and ITS-3N and ITS4 for ITS 2 (Spalik and Downie 2006).
The rpoB-trnC spacer was amplified with primers rpoB and trnCGCAR (Shaw et al. 2005). Because this region includes long insertions and mononucleotide repeats, three internal primers (rpoB400, 400trnC, and 400trnC2) were additionally used to aid amplification and sequencing (Banasiak et al. 2016). The rps16 intron was amplified and sequenced using external primers 5exonC and 3exonR (Calviño et al. 2006) or s16exF and s16exR (Panahi et al. 2015); additionally, two internal primers, s16inF and s16inR, were also used to overcome amplification or sequencing difficulties (Panahi et al. 2015). The rpoC1 intron was amplified and sequenced using external primers C1exF and C1exR; additionally, two internal primers, C1inF and C1inR, were also employed for some samples (Panahi et al. 2015). The sequences were assembled and edited using SeqMan Pro v.14 (Dnastar, Madison, Wisconsin, USA). All newly obtained sequences have been deposited in GenBank (Online Resource 1).
Phylogenetic analyses
Sequences were aligned using the G-INS-I algorithm implemented in MAFFT v.7.271 (Katoh and Standley 2013) and corrected manually as necessary using Mesquite v.3.51 (Maddison and Maddison 2018). The alignment was trimmed with trimAl v.1.2rev59 (Capella-Gutiérrez et al. 2009). Phylogenetic trees were obtained using maximum likelihood (ML) and Bayesian inference (BI) methods. ML analyses were performed with RAxML v.8.2.4 (Stamatakis 2014) employing a GTR + G substitution model with independent estimation of its parameters for nuclear and plastid data. Bootstrap support (BS) was estimated based on 1000 rapid replicate analyses.
Bayesian analyses were performed with MrBayes v.3.2.6 x64 MPI (Ronquist et al. 2012) with GTR + G substitution model with independent estimation of its parameters for nuclear and plastid data. Because in preliminary analyses Ferulinae were paraphyletic with respect to Scandicinae probably due to long-branch attraction, we confined each of the subtribes to monophyly. Two independent runs each of six Monte Carlo Markov chains were initiated and run for 25 million generations with a sampling frequency of 1000 generations. The initial 25% of saved trees were discarded as a burn-in phase; the remaining trees were used to calculate the 50% majority-rule consensus tree and posterior probabilities (PP) of particular clades. To check the effective sample size (ESS) for the estimated parameters and the convergence of the independent runs, Tracer v.1.6.0 (Rambaut et al. 2014) was used.
Morphological analyses
For a few species, we could not assess some quantitative characters because they were not visible in mature fruits due to tissue compression or because the fruits were not fully ripe. Because missing data are problematic in statistical analyses, we filled these gaps using the phylopars function, as implemented in the Rphylopars package v.0.2.9 (Bruggeman et al. 2009). To assess whether there are distinct fruit morphotypes, i.e., groups of morphologically similar fruits, we performed principal component analysis (PCA) as implemented in the R Stats package v.3.5.1 (R Core Team 2018) for qualitative characters (Nos. 1–17, Table 1). The results were visualized using R packages ggplot2 (Wickham 2016) and ggfortify (Tang et al. 2016). To evaluate the evolution of discrete characters (Nos. 18–27, Table 1), we mapped them onto a ML tree using the maximum parsimony method with Mesquite v.3.51 (Maddison and Maddison 2018).
All matrices of molecular and morphological data and their resulting phylogenetic trees were deposited in TreeBASE, study no. 24058 http://purl.org/phylo/treebase/phylows/study/TB2:S24058.
Results
Molecular trees
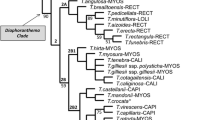
Trees obtained using ML and BI methods were generally similar, with differences including minor rearrangements among some clades or species within these clades (Fig. 2 and Online Resource 2). Major clades—subtribes and genera—were similar to those apparent in former analyses of Scandicinae (Downie et al. 2000a), Ferulinae (Panahi et al. 2018), and Daucinae (Banasiak et al. 2016).
Within Scandicinae, Todaroa aurea, endemic to the Canary Islands and the only anemochorous species in this subtribe, was sister to the barochorous or weakly zoochorous species Athamanta montana, another endemic of this archipelago (BS = 91%; PP = 0.87). Artedia squamata, a species with idiosyncratic fruit morphology, was sister to Daucinae in ML analyses albeit with poor bootstrap support (BS < 50%) while in BI analyses it was placed sister to Torilidinae (PP = 0.71). Most genera of Daucinae that were represented by at least two species or subspecies, i.e., Thapsia, Orlaya, Silphiodaucus, and Daucus, were each monophyletic. Laserpitium included two lineages, with Laserpitium pseudomeum placed sister to Siler montanum (BS = 58%; PP = 0.65) rather than with the rest of its congeners. Generally, the relationships among all major clades of Scandiceae were poorly supported with BS < 50% and PP ≤ 0.71. The lineages with fruits with secondary ribs—Daucinae, Torilidinae, and Artedia—did not form a clade.
Principal component analysis of quantitative traits
In the PCA, the first five components had eigenvalues > 1.0 and together they explained 68.2% of the variance of quantitative morphological characters. The first three principal components (PC1, PC2, and PC3) accounted for 23.2%, 18.8%, and 11.5% of the variance, respectively. The loadings (r) of particular traits for PC1, PC2, and PC3 were low (|r| < 0.5). Dispersal strategies, as estimated based on fruit appendages, were best resolved with PC1; however, they did not correspond to distinct morphotypes because their convex hulls overlapped considerably (Fig. 3). The characters with highest absolute loadings for PC1 included ratio of endosperm groove to endosperm thickness (No. 14, r = − 0.38), ratio of mericarp width 2 to its thickness in soaked fruit (No. 3, r = − 0.37), ratio of mericarp width 1 to its thickness in dry fruit (No. 2, r = − 0.36), and ratio of commissural bundle thickness to its width (No. 9, r = − 0.36). The following characters had lower loadings: ratio of minimum to maximum endosperm groove (No. 15, r = 0.29), ratio of commissure width to mericarp width 2 (No. 12, r =0.26), and approximate plant height (No. 17, r = − 0.25). In summary, anemochorous species tend to have dorsally flattened mericarps with associated changes to their fruit anatomy; they are also somewhat taller than species having other modes of dispersal. This effect is, however, small given low loadings of these traits in PC1 and the low percent of the variation explained by this component. Moreover, the species with lowest negative eigenvalues for PC1 are representatives of Ferulinae. These species have distinctly flattened mericarps with wings formed by primary commissural ribs (hence their commissural bundles are thicker and narrower). This suggests that the variation explained with PC1 may be phylogenetic rather than resulting from similar adaptive shifts in unrelated species.
Ordination plots for PC1 vs. PC2 and PC1 vs. PC3 for 17 quantitative characters of 86 representatives of umbellifers. Different shapes designate groups identified in Fig. 2. Convex hulls with color shading reflect presumed dispersal strategies estimated based on fruit appendages. Numbers of eigenvectors are the same as numbers of characters in Table 1
The evolution of qualitative traits
Phylogenetic mapping of ten qualitative traits on the best ML tree demonstrated that all fruit characters are homoplastic and of limited use for defining taxa; they may, however, reflect convergent adaptive evolution (Fig. 4). Wings on lateral primary ribs developed in three ingroup lineages: Ferulinae, Todaroa aurea (Scandicinae) and Laser trilobum (Daucinae). Secondary ribs arose twice, in the ancestor of Torilidinae and the common ancestor of Daucinae and Artedia. However, because basal relationships within Scandiceae are poorly supported, a unique appearance of this character cannot be excluded.
Spiny secondary ribs are ancestral for Torilidinae, while winged ribs are ancestral for Daucinae. In Torilidinae, wings evolved from spines in Lisaea papyracea, while in Daucinae spines evolved from wings likely in the common ancestor of Orlaya, Silphiodaucus, and Daucus with subsequent reversals in Silphiodaucus, in the ancestor of Daucus edulis and D. decipiens, in D. rouyi, and in D. annuus (but note the ambiguity of reconstruction). Wings of Artedia are idiosyncratic as they occur only on lateral secondary ribs and are divided into broad, round lobes. It is noteworthy that 22 species are annuals and distinctly zoochorous, i.e., have spiny fruits, as opposed to seven annuals without spiny fruits and five spiny-fruited taxa that are biennials or perennials (Fig. 5). However, this co-occurrence is due to common ancestry rather than to convergence or parallel evolution because the ancestors of Torilidinae, Orlaya, and Daucus are annuals with spiny fruits.
Laserpitium sensu Banasiak et al. (2016) is characterized by the presence of non-elongated sclerified cells in endocarp and mesocarp tissues. This character also occurs in Ekimia petrophila, but not in Laserpitium pseudomeum. Fruits of Thapsia sensu Banasiak et al. (2016) usually have 6–10 or more vittae with some vittae situated under vascular bundles; both characters are, however, highly homoplastic.
Discussion
Fruit morphologies and dispersal modes in Apiaceae
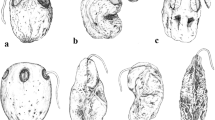
The results suggest that there are no clear-cut morphological dispersal syndromes in Apiaceae tribe Scandiceae, but a wide spectrum of fruit morphotypes (Fig. 6) that may reflect diverse and probably mixed dispersal strategies. Due to their small fruit size, Apiaceae are opportunistic dispersers and their actual dispersal mode may depend on local vectors while their fruit morphology may sometimes be misleading. On the other hand, its highly homoplastic nature suggests that fruit morphology in Apiaceae is subject to strong selective pressure.
Experimental studies demonstrate that winged mericarps of umbellifers are carried away by wind a relatively short distance: 90% of fruits reached the ground within five meters from the maternal plant (Jongejans and Telenius 2001). Therefore, other vectors must be responsible to account for their long-distance dispersal. Our results suggest that winged fruits more often occur in tall, robust umbellifers, such as the several species of Ferula, Laserpitium, and Thapsia examined herein. However, this morphology is better seen as a way to avoid competition of seedlings with the maternal plant than as a means for long-distance dispersal. A study on the presumably wind-dispersed giant hogweed (Heracleum mantegazzianum) demonstrated that the maximum distance of seedlings from the maternal plant was 5.3 m, with the highest density of seedlings within the range 0.0–0.5 m (Pergl et al. 2011). Modeling of a regional invasion of H. mantegazzianum in the Czech Republic suggested that short-distance dispersal (neighboring dispersal) up to 10 m does not explain its spread; therefore, long-distance dispersal (10–500 m) by other vectors has had to take place (Nehrbass et al. 2006; Pergl et al. 2011). A study on animal dispersal has shown that some presumed wind-dispersed and gravity-dispersed umbellifer fruits have high attachment potential (AtP) to animal fur (Römermann et al. 2005). Fruit morphology and fruit size were good predictors of attachment potential to sheep fur, with spiny Daucus carota and winged Laserpitium latifolium having AtP values of 95.7% and 13.7%, respectively. However, the difference disappeared with cattle as dispersing agents (AtP values of 16.7% and 10.3%, respectively) and this time fruit mass alone was the best predictor of attachment potential. Diaspores above a threshold of about 10 mg did not keep attached to cattle hair at all (Römermann et al. 2005). Effectively, spines may serve both epizoochory and anemochory. In a wind-dispersal experiment including fruits of D. carota with artificially removed spines, spineless fruits went only two-thirds as far as the intact diaspores even though the former weighed less than the latter (Lacey 1981).
As compared to winged fruits, epizoochorous fruits are generally dispersed at higher distances. In a study modeling seed shade for epizoochorous dispersal by donkeys including the umbellifer Anthriscus caucalis, mean dispersal distance for hooked fruits was estimated at 166 m (Couvreur et al. 2008).
A good example of contradictory selection pressures acting on dispersal syndromes is heterocarpy: the presence of different diaspore morphotypes on a single plant reflecting various modes of dispersal. Among umbellifers, barochorous and epizoochorous fruits occur in Torilis nodosa, Lisaea heterocarpa, Tordylium aegyptiacum, Scandix australis, and S. turgida (Arenas Posada and García Martín 1993; Hedge and Lamond 1972; Jury 1986). It is noteworthy that all of these species are annuals. Barochorous fruits serve therefore to maintain the local population, while epizoochorous diaspores are adapted for long-distance dispersal.
When inferring dispersal strategies from morphology, in some cases the entire plant habit must also be taken into account. For example, 16% of umbellifers native to Israel are tumbleweeds (Cohen and Plitmann 1997); hence, the entire plant is the dispersal unit. In some species of Daucus, including D. carota, rays of fruiting umbels are bent inward forming a bird’s nest structure. These rays bend adaxially with increasing relative humidity and abaxially with decreasing relative humidity, affecting fruit dispersal (Lacey 1980; Lacey et al. 1983). Conventional plant dispersal classification systems based on diaspore appendages are therefore of limited use for addressing ecological questions because the dispersal potential ranges continually (Tackenberg et al. 2003).
Fruit character evolution in Daucinae
In Apiaceae, prominent fruit secondary ribs are unique to three major lineages of Scandiceae—subtribes Daucinae and Torilidinae and the genus Artedia—suggesting that this character is a synapomorphy uniting these three clades. However, these lineages formed a clade neither in our study nor in previously published molecular trees of the group (Downie et al. 2000a; Lee and Downie 1999; Lee et al. 2001). Nevertheless, in all of these analyses, the relationships among major clades were poorly supported and prone to taxon sampling. Most recent RAD-seq analyses of Scandiceae and outgroups strongly supported a sister relationship between Daucinae and Torilidinae and, correspondingly, synapomorphic status of prominent secondary ribs (Marcin Piwczyński, unpubl.).
Spiny-fruited taxa of Daucinae and Torilidinae were formerly placed in tribe Caucalideae while winged-fruited taxa were classified in tribe Laserpitieae. However, both previous analyses (Banasiak et al. 2016) and this study demonstrated that the morphology of fruit appendages is highly homoplastic. The presence of wings or spines is of limited use at the generic level. Particularly, the genus Daucus has been redefined to include winged-fruited Melanoselinum decipiens (≡ D. decipiens), Monizia edulis (≡ D. edulis), Rouya polygama (≡ D. rouyi), and the species of Tornabenea that were partly transferred to Daucus: D. annuus, D. tenuissimus, D. insularis (Banasiak et al. 2016), with the first included in this study. In fact, the appendages of secondary ribs in D. annuus are intermediate between wings and spines. The evolution of winged fruits in these taxa is most likely a reversal, and in some cases, it may be linked to their insular habitat. Daucus decipiens and D. edulis are endemic to Madeira (Fernandes and Carvalho 2014; Press and Dias 1998), while Tornabenea is restricted to Cape Verde (Brochmann et al. 1997). These volcanic Macaronesian archipelagos have never been connected to the mainland, and they lack native large mammals that could have acted as dispersing agents for epizoochorous species. Particularly interesting are the changes that occurred in ‘Tornabenea,’ exemplified in this study by D. annuus. This species is nested within the D. carota complex, and its evolution was apparently recent. It has spiny or dentate lateral wings indicating that they are derived from spines of D. carota. Additionally, its reduced dorsal secondary ribs are similar to the two endemics of Madeira illustrating, therefore, evolutionary parallelism. In our former molecular study, Tornabenea was placed under synonymy in Daucus (Banasiak et al. 2016); however, some species were not included in that study and thus not formally transferred making the classification system inconsistent. We, therefore, introduce new combinations in Daucus below.
Due to numerous homoplasies, fruit characters are of limited utility for delineating generic boundaries in Daucinae as none of the genera is defined based on a synapomorphy. Some genera, however, are defined based on sets of characters, such as the segregates of Laserpitium. All species traditionally placed in Laserpitium are characterized by the presence of wings at all secondary ridges. However, Laserpitium sensu Banasiak et al. (2016) or sensu stricto (s.s.) may be distinguished from its former congeners now placed in Ekimia, Laser, and Thapsia by a suite of characters including the shape of endosperm in transverse section and the fruit sclerification pattern. Particularly interesting are the differences between the species of Laserpitium s.s. and L. pseudomeum, the latter constituting an isolated lineage that may be sister to Siler (this study) or to Laser (Banasiak et al. 2016) rather than to its congeners. Fruits of L. pseudomeum are superficially similar to Laserpitium s.s. in having prominent wings both on dorsal and lateral secondary ribs but are more similar to Laser in having distinctly flattened endosperm and non-sclerified exocarp and mesocarp. Laserpitium pseudomeum is endemic to the mountain regions of Greece (Hartvig 1986). Additional molecular studies are necessary to firmly establish its phylogenetic position.
The phylogenetic position of A. squamata was unstable in previous molecular analyses. Similar to this study, it was placed sister to Daucinae (Lee and Downie 1999, 2000) or Ferulinae (Banasiak et al. 2013) or identified as basal branch in Scandiceae (Valiejo-Roman et al. 2006). Given its peculiar fruit morphology, pollen traits (Cerceau-Larrival and Roland-Heydacker 1976), and isolated phylogenetic position, it deserves to be placed in a separate subtribe.
Conclusions
Our results suggest that in Scandiceae, and Daucinae in particular, there are no clear-cut dispersal syndromes. Only anemochory is weakly associated with mericarp flattening and concomitant changes in fruit anatomy like endosperm shape. These associations are weak because fruit appendages facilitating wind dispersal may be of different origins, such as developing from either primary or secondary ribs. It is, however, noteworthy that multiple switches between wings and spines occurred only in taxa where these appendages occur on secondary ribs. To our knowledge, there are no spines—that have to be distinguished from epidermal bristles—developing from primary ribs. Primary ribs contain vascular bundles that may constrain such a transformation.
Similar to previous studies, our study demonstrates that fruit morphology is highly homoplastic in umbellifers and that synapomorphies that are useful for infrafamilial classification rarely occur.
Taxonomic treatment
Daucus bischoffii (J.A.Schmidt) Spalik & Banasiak, comb. nov. ≡ Tornabenea bischoffii J.A.Schmidt, Beitr. Fl. Cap Verd. Ins. 254. 1852.—LECTOTYPE: Cape Verde, Santo Antão, mountain screes, Mar 1861, J.A. Schmidt s.n. (Brochmann et al. 1997, W [n.v.]).
Daucus humilis (Lobin & K.H.Schmidt) Spalik & Wojew., comb. nov. ≡ Tornabenea humilis Lobin & K.H.Schmidt, Sommerfeltia 24: 83. 1997.—HOLOTYPE: Fogo, 8 Dec 1982, Lobin 2621 (FR [n.v.]).
Daucus ribeirensis (K.H.Schmidt & Lobin) Spalik & Baczyński, comb. nov. ≡ Tornabenea ribeirensis K.H.Schmidt & Lobin, Feddes Repert. 110(1–2): 8. 1999. —HOLOTYPE: São Nicolau, 25 Nov 1982, Lobin 2562 (B [n.v.]).
Artediinae Baczyński, Wojew., S.R.Downie & Spalik subtrib. nov. —TYPE: Artedia L., Sp. Pl. 1: 242. 1753.
Diagnosis: Inflorescence with central dark florets on elongated stalks; fruits with broadly lobed wings on lateral secondary ribs; pollen distinctly rhomboidal with exceptionally strong apiculation (protrusion) above aperture. Monotypic.
Distribution area: Eastern Mediterranean to Middle East.
References
Arenas Posada JA, García Martín F (1993) Atlas carpológico y corológico de la subfamilia Apioideae Drude (Umbelliferae) en España peninsular y Baleares. Ruizia 12:1–245
Banasiak Ł, Piwczyński M, Uliński T, Downie SR, Watson MF, Shakya B, Spalik K (2013) Dispersal patterns in space and time: a case study of Apiaceae subfamily Apioideae. J Biogeogr 40:1324–1335. https://doi.org/10.1111/jbi.12071
Banasiak Ł, Wojewódzka A, Baczyński J, Reduron J-P, Piwczyński M, Kurzyna-Młynik R, Gutaker R, Czarnocka-Cieciura A, Kosmala-Grzechnik S, Spalik K (2016) Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. Taxon 65:563–585. https://doi.org/10.12705/653.8
Brochmann C, Rustan OH, Lobin W, Kilian N, Schmidt KHA (1997) The endemic vascular plants of the Cape Verde. Tornabenea Parl. Sommerfeltia 24:77–91
Bruggeman J, Heringa J, Brandt BW (2009) PhyloPars: estimation of missing parameter values using phylogeny. Nucl Acids Res 37(suppl. 2):W179–W184. https://doi.org/10.1093/nar/gkp370
Calviño CI, Tilney PM, van Wyk BE, Downie SR (2006) A molecular phylogenetic study of southern African Apiaceae. Amer J Bot 93:1828–1847. https://doi.org/10.3732/ajb.93.12.1828
Capella-Gutiérrez S, Silla-Martínez JM, Gabaldón T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25:1972–1973. https://doi.org/10.1093/bioinformatics/btp348
Cerceau-Larrival MT, Roland-Heydacker F (1976) Ontogénie et ultrastructure de pollens d'Ombellifères. Tapis et corps d'Ubisch. C R Acad Sci Paris 283:29–32
Cohen O, Plitmann U (1997) Dispersal strategies in the Apiaceae: the temporal factor and its role in dissemination. Lagascalia 19:423–438
Couvreur M, Verheyen K, Vellend M, Lamoot I, Cosyns E, Hoffmann M, Hermy M (2008) Epizoochory by large herbivores: merging data with models. Basic Appl Ecol 9:204–212. https://doi.org/10.1016/j.baae.2006.12.002
Downie SR, Katz-Downie DS, Spalik K (2000a) A phylogeny of Apiaceae tribe Scandiceae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. Amer J Bot 87:76–95
Downie SR, Katz-Downie DS, Watson MF (2000b) A phylogeny of the flowering plant family Apiaceae based on chloroplast DNA rpl16 and rpoC1 intron sequences: towards a suprageneric classification of subfamily Apioideae. Amer J Bot 87:273–292
Downie SR, Spalik K, Katz-Downie DS, Reduron JP (2010) Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Pl Diversity Evol 128:111–136. https://doi.org/10.1127/1869-6155/2010/0128-0005
Fernandes F, Carvalho JA (2014) An historical review and new taxa in the Madeiran endemic genus Monizia (Apiaceae, Apioideae). Webbia 69:13–37. https://doi.org/10.1080/00837792.2014.909648
Grushvitsky IV, Tikhomirov VN, Aksenov ES, Shibakina GV (1969) Succulent fruit with carpophore in species of the genus Stilbocarpa Decne. et Planch. (Araliaceae). Byull Moskovsk Obshch Isp Prir Otd Biol 74:64–76 (in Russian)
Harper JL, Lovell PH, Moore KG (1970) The shapes and sizes of seeds. Annual Rev Ecol Evol Syst 1:327–356
Hartvig P (1986) Apiaceae (Umbelliferae). In: Strid A (ed) Mountain flora of Greece, vol. 1. Cambridge University Press, Cambridge, pp 655–735
Hedge IC, Lamond JM (1972) Scandix L. In: Davis PH (ed) Flora of Turkey and the East Aegean Islands, vol. 4. Edinburgh University Press, Edinburgh, pp 325–330
Heywood VH (1982) Multivariate taxonomic synthesis of the tribe Caucalideae. In: Cauwet-Marc AM, Carbonnier J (eds) Actes du 2ème Symposium international sur les Ombellifères “Contributions pluridisciplinaires à la systématique”. Braun-Brumfield, Ann Arbor, pp 727–736
Heywood VH, Dakshini KMM (1971) Fruit structure in the Umbelliferae-Caucalideae. In: Heywood VH (ed) The biology and chemistry of the Umbelliferae. Academic Press, London, pp 215–232
Jongejans E, Telenius A (2001) Field experiments on seed dispersal by wind in ten umbelliferous species (Apiaceae). Pl Ecol 152:67–78. https://doi.org/10.1023/A:1011467604469
Jury SL (1986) Fruit and leaf variation in the African species of the Umbelliferae tribe Caucalideae. Symb Bot Upsal 26:181–188
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molec Biol Evol 30:772–780. https://doi.org/10.1093/molbev/mst010
Korovin EP (1947) Generis Ferula (Tourn.) L. monographia illustrata. Academiae Scientiarum UzRSS, Tashkent
Kurzyna-Młynik R, Oskolski AA, Downie SR, Kopacz R, Wojewódzka A, Spalik K (2008) Phylogenetic position of the genus Ferula (Apiaceae) and its placement in tribe Scandiceae as inferred from nrDNA ITS sequence variation. Pl Syst Evol 274:47–66. https://doi.org/10.1007/s00606-008-0022-2
Lacey EP (1980) The influence of hygroscopic movement on seed dispersal in Daucus carota (Apiaceae). Oecologia 47:110–114. https://doi.org/10.2307/4216207?ref=search-gateway:6d2ecb38638b3cd2c4273504448e436c
Lacey EP (1981) Seed dispersal in wild carrot (Daucus carota). Michigan Bot 20:15–20
Lacey EP, Kaufman PB, Dayanandan P (1983) The anatomical basis for hygroscopic movement in primary rays of Daucus carota ssp. carota (Apiaceae). Bot Gaz 144:371–375
Lee BY, Downie SR (1999) A molecular phylogeny of Apiaceae tribe Caucalideae and related taxa: inferences based on ITS sequence data. Syst Bot 24:461–479. https://doi.org/10.2307/2419700
Lee BY, Downie SR (2000) Phylogenetic analysis of cpDNA restriction sites and rps16 intron sequences reveals relationships among Apiaceae tribes Caucalideae, Scandiceae and related taxa. Pl Syst Evol 221:35–60. https://doi.org/10.1007/BF01086379
Lee BY, Levin GA, Downie SR (2001) Relationships within the spiny-fruited umbellifers (Scandiceae subtribes Daucinae and Torilidinae) as assessed by phylogenetic analysis of morphological characters. Syst Bot 26:622–642
Liu M, Plunkett GM, Lowry PP, van Wyk BE, Tilney PM (2006) The taxonomic value of fruit wing types in the order Apiales. Amer J Bot 93:1357–1368. https://doi.org/10.3732/ajb.93.9.1357
Maddison WP, Maddison DR (2018) Mesquite: a modular system for evolutionary analysis. Version 3.51. Available at: http://mesquiteproject.org
Nehrbass N, Winkler E, Müllerová J, Pergl J, Pyšek P, Perglová I (2006) A simulation model of plant invasion: long-distance dispersal determines the pattern of spread. Biol Invas 9:383–395. https://doi.org/10.1007/s10530-006-9040-6
Panahi M, Banasiak Ł, Piwczyński M, Puchałka R, Oskolski AA, Spalik K (2015) Phylogenetic relationships among Dorema, Ferula and Leutea (Apiaceae, Scandiceae, Ferulinae) inferred from nrDNA ITS and cpDNA noncoding sequences. Taxon 64:770–783. https://doi.org/10.12705/644.8
Panahi M, Banasiak Ł, Piwczyński M, Puchałka R, Kanani MR, Oskolski AA, Modnicki D, Miłobędzka A, Spalik K (2018) Taxonomy of the traditional medicinal plant genus Ferula (Apiaceae) is confounded by incongruence between nuclear rDNA and plastid DNA. Bot J Linn Soc 188:173–189
Pergl J, Müllerová J, Perglová I, Herben T, Pyšek P (2011) The role of long-distance seed dispersal in the local population dynamics of an invasive plant species. Diversity Distrib 17:725–738. https://doi.org/10.1111/j.1472-4642.2011.00771.x
Piwczyński M, Puchałka R, Spalik K (2015) The infrageneric taxonomy of Chaerophyllum (Apiaceae) revisited: new evidence from nuclear ribosomal DNA ITS sequences and fruit anatomy. Bot J Linn Soc 178:298–313. https://doi.org/10.1111/boj.12282
Press JR, Dias E (1998) The genera Melanoselinum Hoffm. and Angelica L. (Umbelliferae) in Macaronesia. Arquipélago, Life Mar Sci 16A:1–10
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. Available at: http://www.R-project.org/
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6. Available at: http://beast.bio.ed.ac.uk/Tracer
Römermann C, Tackenberg O, Poschlod P (2005) How to predict attachment potential of seeds to sheep and cattle coat from simple morphological seed traits. Oikos 110:219–230
Ronce O, Clobert J (2012) Dispersal syndromes. In: Clobert J, Baguette M, Benton TG, Bullock JM (eds) Dispersal ecology and evolution. Oxford University Press, Oxford, pp 119–138
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Shaw J, Lickey EB, Beck JT, Farmer SB, Liu W, Miller J, Siripun KC, Winder CT, Schilling EE, Small RL (2005) The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analyses. Amer J Bot 92:142–166. https://doi.org/10.3732/ajb.92.1.142
Spalik K, Downie SR (2006) The evolutionary history of Sium sensu lato (Apiaceae): dispersal, vicariance, and domestication as inferred from ITS rDNA phylogeny. Amer J Bot 93:747–761. https://doi.org/10.3732/ajb.93.5.747
Spalik K, Wojewódzka A, Downie SR (2001) The evolution of fruit in Scandiceae subtribe Scandicinae (Apiaceae). Canad J Bot 79:1358–1374. https://doi.org/10.1139/b01-116
Spalik K, Reduron JP, Downie SR (2004) The phylogenetic position of Peucedanum sensu lato and allied genera and their placement in tribe Selineae (Apiaceae, subfamily Apioideae). Pl Syst Evol 243:189–210. https://doi.org/10.1007/s00606-003-0066-2
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. https://doi.org/10.1093/bioinformatics/btu033
Tackenberg O, Poschlod P, Bonn S (2003) Assessment of wind dispersal potential in plant species. Ecol Monogr 73:191–205
Tackenberg O, Römermann C, Thompson K, Poschlod P (2006) What does diaspore morphology tell us about external animal dispersal? Evidence from standardized experiments measuring seed retention on animal-coats. Basic Appl Ecol 7:45–58. https://doi.org/10.1016/j.baae.2005.05.001
Tang Y, Horikoshi M, Li W (2016) ggfortify: unified interface to visualize statistical result of popular R packages. R Journal 8:474–485
Valiejo-Roman CM, Terentieva EI, Samigullin TH, Pimenov MG, Ghahremani-Nejad F, Mozaffarian V (2006) Molecular data (nrITS-sequencing) reveal relationships among Iranian endemic taxa of Umbelliferae. Feddes Repert 117:367–388. https://doi.org/10.1002/fedr.200611106
Weitzel C, Rønsted N, Spalik K, Simonsen HT (2014) Resurrecting deadly carrots: towards a revision of Thapsia (Apiaceae) based on phylogenetic analysis of nrITS sequences and chemical profiles. Bot J Linn Soc 174:620–636. https://doi.org/10.1111/boj.12144
Wen J, Zimmer EA (1996) Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Molec Phylogen Evol 6:167–177. https://doi.org/10.1006/mpev.1996.0069
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Williams CF (1994) Genetic consequences of seed dispersal in three sympatric forest herbs. II. Microspatial genetic structure within populations. Evolution 48:1959–1972. https://doi.org/10.2307/2410520
Williams CF, Guries RP (1994) Genetic consequences of seed dispersal in three sympatric forest herbs. I. Hierarchical population-genetic structure. Evolution 48:791–805. https://doi.org/10.2307/2410487
Winter PJD, Magee AR, Phephu N, Tilney PM, Downie SR, van Wyk BE (2008) A new generic classification for African peucedanoid species (Apiaceae). Taxon 57:347–364
Yu Y, Downie SR, He X, Deng X, Yan L (2011) Phylogeny and biogeography of Chinese Heracleum (Apiaceae tribe Tordylieae) with comments on their fruit morphology. Pl Syst Evol 296:179–203. https://doi.org/10.1007/s00606-011-0486-3
Acknowledgements
Access to the material was kindly provided by the curators of the herbaria and the staff of the botanical institutions listed in Online Resource 1. We are particularly grateful to Jean-Pierre Reduron and the late Pedro Montserrat for providing ripe fruits of some hardly accessible species. The research was funded by the Polish Ministry of Science and Higher Education Grant N N303 069335, the National Science Center Grant 2015/19/B/NZ8/00163 to KS, and 2014/15/D/NZ8/00286 to ŁB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Ferhat Celep.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Information on electronic supplementary material
Information on electronic supplementary material
Online Resource 1. Accessions of Apiaceae examined for fruit morphology and DNA sequence variation.
Online Resource 2. Bayesian 50% majority-rule consensus tree for 86 representatives of Apiaceae tribe Scandiceae and outgroups.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wojewódzka, A., Baczyński, J., Banasiak, Ł. et al. Evolutionary shifts in fruit dispersal syndromes in Apiaceae tribe Scandiceae. Plant Syst Evol 305, 401–414 (2019). https://doi.org/10.1007/s00606-019-01579-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-019-01579-1