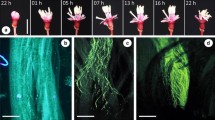
Abstract
The evolution of dioecy from heterostyly has been well documented, but detailed studies on this transitional process are rare. Here we report the occurrence of cryptic dioecy in a perennial liana species with stigma-height dimorphism, Morinda parvifolia Bartl. ex DC. (Rubiaceae). Floral morphology, ancillary characters and cross compatibility of long-styled (L-morph) and short-styled (S-morph) were examined. L-morph and S-morph display obvious pistil dimorphisms, with the stigma of S-morph lacking papillae cells. Both floral morphs show similar pollen morphology, although pollen viability is higher in S-morph than in L-morph. S-morph flowers produce viable pollen grains but much reduced stigma and set no fruits, functioning as males; L-morphs, although with viable pollen grains and receptive stigmas, exhibit strong self- and intramorph incompatibility, with self- and intramorph pollen tubes arrested in the stigma lobes and the upper part of style, respectively, resulting in L-morphs functioning only as females. The species thus has physiological androdioecy but functional dioecy. This might be the first case showing the possibility that androdioecy could be a mid-stage in the pathway of dioecy evolving from stigma-height dimorphism.



Similar content being viewed by others
References
Bahadur B (1968) Heterostyly in Rubiaceae: a review. J Osman Univ (Sci) Golden Jubilee Volume: 207–238
Bahadur B (1970) Heterostyly in Hedyotis nigricans (Lam.) Fosb. J Genet 60(2):175–177
Baker HG (1958) Studies in reproductive biology of West African Rubiaceae. J West Afric Sci Assoc 4:4–24
Baker CA, Bakhiuzen RCVDB (1965) Angiospermae. In: Flora of Java, vol 2. Noordhoff NVP, Netherlands
Baker AM, Thompson JD, Barrett SCH (2000) Evolution and maintenance of stigma-height dimorphism in Narcissus. I Floral variation and style-morph ratios. Heredity 84:502–513
Barrett SCH, Shore JS (2008) New Insights on Heterostyly: Comparative Biology, Ecology and Genetics. In: Franklin-Tong VE (ed) Self-incompatibility in flowering plants—evolution, diversity, and mechanisms. Springer, Berlin, pp 3–32
Bawa KS (1980) Evolution of dioecy in flowering plants. Annu Rev Ecol Syst 11:15–39
Beach JH, Bawa KS (1980) Role of pollinators in the evolution of dioecy from distyly. Evolution 34(6):1138–1142
Bentham G, Hooker JD (1876) Genera Plantarum, vol 2. London
Bremekamp CEB (1966) Remarks on the position, the delimitation and the subdivision of the Rubiaceae. Acta Bot Neerlandica 15:1–33
Bremer B, Manen JF (2000) Phylogeny and classification of the subfamily Rubioideae (Rubiaceae). Plant Syst Evol 225:43–72
Bremer B, Andreasen K, Olsson D (1995) Subfamilial and tribal relationships in the Rubiaceae based on rbcL sequence data. Ann Missouri Bot Gard 82:383–397
Bremer B, Jansen RK, Oxelman B, Backlund M, Lantz H, Kim K-J (1999) More characters or more taxa for a robust phylogeny—case study from the coffee family (Rubiaceae). Syst Biol 48(3):413–435
Burck MW (1883) Sur 1’organisation florale chez quelques Rubiacees. Ann Jard Bot Buitenz 3:105–119
Castro CC, Oliveira PEAM, Alves MC (2004) Breeding system and floral morphometry of distylous Psychotria L. species in the Atlantic rain forest, SE Brazil. Plant Biol 6:755–760
Charlesworth D (1984) Androdioecy and the evolution of dioecy. Biol J Linn Soc 23:333–348
Charlesworth D (1999) Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LE (eds) Gender and sexual dimorphism in flowering plants. Springer, New York, pp 33–60
Charlesworth B, Charlesworth D (1978a) A model for the evolution of dioecy and gynodioecy. Am Nat 112:975–997
Charlesworth D, Charlesworth B (1978b) Population-genetics of partial male-sterility and evolution of monoecy and dioecy. Heredity 41:137–153
Dafni A (1992) Pollination ecology: a practical approach. Oxford University Press, New York
Darwin CR (1877) The different forms of flowers and plants of the same species. John Murray, London
Dommée B, Bompar JL, Denelle N (1990) Sexual tetramorphism in Thymelaea hirsuta (Thymelaeaceae): evidence of the pathway from heterodichogamy to dioecy at the infraspecific level. Am J Bot 77:1449–1462
Dorken ME, Friedman J, Barrett SCH (2002) The evolution and maintenance of monoecy and dioecy in Sagittaria latifolia (Alismataceae). Evolution 56:31–41
Dulberger R (1992) Floral polymorphisms and their functional significance in the heterostylous syndrome. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer, Berlin, pp 41–77
East EM (1940) The distribution of self sterility in flowering plants. Proc Am Phil Soc 82:449–518
Eckert CG, Barrett SCH (1994) Tristyly, self-compatibility and floral variation in Decodon verticillatus (Lythraceae). Biol J Linn Soc 53:1–30
Erdtman G (1952) Pollen morphology and plant taxonomy: angiosperms. Almqvist and Wiksell Press, Stockholm
Faegri K, Iversen J (1964) Textbook of pollen analysis. Munksgaard Press, Copenhagen
Faivre AE, Mcdade LA (2001) Population-level variation in the expression of heterostyly in three species of Rubiaceae: does reciprocal placement of anthers and stigmas characterize heterostyly? Am J Bot 88(5):841–853
Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L (2009) Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae). Persp Plant Eco Evol Syst 11:111–125
Ganders FR (1979) The biology of heterostyly. New Zeal J Bot 17:607–635
Gleiser G, Verdú M (2005) Repeated evolution of dioecy from androdioecy in Acer. New Phytol 165:633–640
Johansson JT (1994) The genus Morinda (Morindeae, Rubioideae, Rubiaceae) in New Caledonia: taxonomy and phytogeny. Opera Bot 122:1–67
Lepart J, Dommée B (1992) Is Phillyrea angustifolia L. (Oleaceae) an androdioecious species? Bot J Linn Soc 108:375–387
Li AM, Wu XQ, Zhang DX, Barrett SCH (2010) Cryptic dioecy in Mussaenda pubescens (Rubiaceae): a species with stigma-height dimorphism. Ann Bot 106(4):521–531
Liston A, Rieseberg LH, Elias TS (1990) Functional androdioecy in the flowering plant Datisca glomerata. Nature 343:641–642
Liu Y (2010) Taxonomic revision of Morinda in China. Ph. D Dissertation, The Chinese Academy of Sciences, China
Lloyd DG (1974) The genetic contributions of individual males and females in dioecious and gynodioecious angiosperms. Heredity 32:45–51
Lloyd DG (1975) The maintenance of gynodioecy and androdioecy in angiosperms. Genetica 45:325–339
Lloyd DG (1979) Evolution towards dioecy in heterostylous populations. Plant Syst Evol 131:71–80
Lloyd DG (1980) The distributions of gender in four angiosperm species illustrating two evolutionary pathways to dioecy. Evolution 34:123–134
Lloyd DG, Webb CJ (1992a) The evolution of heterostyly. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer, Berlin, pp 151–178
Lloyd DG, Webb CJ (1992b) The selection of heterostyly. In: Barrett SCH (ed) Evolution and function of heterostyly. Springer, Berlin, pp 179–207
Luo SX, Zhang DX, Renner SS (2007) Duodichogamy and androdioecy in the Chinese Phyllanthaceae Bridelia tomentosa. Am J Bot 94(2):260–265
Lush WM, Clarke AE (1997) Observations of pollen tube growth in Nicotiana alata and their implications for the mechanism of self-incompatibility. Sex Plant Reprod 10:27–35
Massinga PH, Johnson SD, Harder LD (2005) Heteromorphic incompatibility and efficiency of pollination in two distylous Pentanisia species (Rubiaceae). Ann Bot 95:389–399
Mayer SS, Charlesworth D (1991) Cryptic dioecy in flowering plants. Trends Ecol Evol 6:320–325
Murfett J, Atherton TL, Mou BQ, Gassert CS, Mcclure BA (1994) S-RNase expressed in transgenic Nicotiana causes S-allele-specific pollen rejection. Nature 367:563–566
Naiki A, Kato M (1999) Pollination system and evolution of dioecy from distyly in Mussaenda parviflora (Rubiaceae). Plant Spec Biol 14:217–227
Naiki A, Nagamasu H (2003) Distyly and pollen dimorphism in Damnacanthus (Rubiaceae). J Plant Res 116:105–113
Pannell JR (2002) The evolution and maintainance of androdioecy. Annu Rev Ecol Syst 33:397–425
Pendleton RL, Freeman C, McArthur ED, Sanderson SC (2000) Gender specialization in heterodichogamous Grayia brandegei (Chenopodiaceae): evidence for an alternative pathway to dioecy. Am J Bot 87:508–516
Pérez R, Vargas P, Arroyo J (2003) Convergent evolution of flower polymorphism in Narcissus (Amaryllidaceae). New Phytol 161:235–252
Philip O, Mathew PM (1978) Heterostyly and pollen dimorphism in Morinda tinctoria Roxb. New Bot 5:47–51
Razafimandimbison SG, Rydina C, Bremer B (2008) Evolution and trends in the Psychotrieae alliance (Rubiaceae)—a rarely reported evolutionary change of many-seeded carpels from one-seeded carpels. Mol Phylogenet Evol 48:207–223
Razafimandimbison SG, McDowell TD, Halford DA, Bremer B (2009) Molecular phylogenetics and generic assessment in the tribe Morindeae (Rubiaceae–Rubioideae): how to circumscribe Morinda L. to be monophyletic? Mol Phylogenet Evol 52:879–886
Reddy NP, Bahadur B (1978) Heterostyly in Morinda tomentosa Roxb. (Rubiaceae). Acta Bot Indica 6:63–70
Renner S, Won H (2001) Repeated evolution of dioecy from monoecy in Siparunaceae (Laurales). Syst Biol 50:700–712
Riveros GM, Barría OR, Humaña PAM (1995) Self-compatibility in distylous Hedyotis salzmannii (Rubiaceae). Pl Syst Evol 194:1–8
Robbrecht E (1988) Tropical woody Rubiaceae. Characteristic features and progressions. Contributions to a new subfamilial classification. Opera Bot Belg 1:1–271
Robbrecht E, Manen JF (2006) The major evolutionary lineages of the coffee family (Rubiaceae, angiosperms). Combined analysis (nDNA and cpDNA) to infer the position of Coptosapelta and Luculia, and supertree construction based on rbcL, rps16, trnL-trnF and atpB-rbcL data. A new classification in two subfamilies Cinchonoideae and Rubioideae. Syst Geog Pl 76:85–145
Rodriguez-Riaño T, Dafni A (2000) A new procedure to assess pollen viability. Sex Plant Reprod 12:241–244
Ruan YZ (1999) Morinda L. In: Chen WQ, Lo HS, Ko WC, Ruan YZ (eds) Flora reipublicae popularis sinicae, vol 71., 2Science Press, Beijing, pp 179–202
Sakai S (2001) Thrips pollination of androdioecious Castilla elastica (Moraceae) in a seasonal tropical forest. Am J Bot 88:1527–1534
Sánchez JM, Ferrero V, Arroyo J, Navarro L (2010) Patterns of style polymorphism in five species of the South African genus Nivenia (Iridaceae). Ann Bot 106:321–331
Scribailo RW, Barrett SCH (1991) Pollen-pistil interactions in tristylous Pontederia sagittata (Pontederiaceae). Floral heteromorphism and structural features of the pollen tube pathway. Am J Bot 78(12):1643–1661
Seger J, Eckhart VM (1996) Evolution of sexual systems and sex allocation in plants when growth and reproduction overlap. Proc R Soc London Ser B 263:833–841
Skotisberg C (1944) On the flower dimorphism in Hawaiian Rubiaceae. Ark f Bot 31:1–28
Sobrevila C, Ramirez N, De Enrech NX (1983) Reproductive biology of Palicourea fendleri and P. petiolaris (Rubiaceae), heterostylous shrubs of a tropical cloud forest in Venezuela. Biotropica 15(3):161–169
Teng NJ, Chen T, Jin B, Wu XQ, Huang ZH, Li XJ, Wang YH, Mu XJ, Lin JX (2006) Abnormalities in pistil development result in low seed set in Leymus chinensis (Poaceae). Flora 201:658–667
Verdcourt B (1958) Remarks on the classification of the Rubiaceae. Bull Jard Bot Brux 28:209–281
Vithanage HIMV, Gleeson PA, Clarke AE (1980) The nature of callose produced during self-pollination in Secale cereale. Planta 148:498–509
Wallander E (2001) Evolution of wind pollination in Fraxinus (Oleaceae): an ecophylogenetic approach. Dissertation, Göteborg University
Wang YQ, Zhang DX, Chen ZY (2004) Pollen histochemistry and pollen: ovule ratios in Zingiberaceae. Ann Bot 94:583–591
Webb CJ (1999) Empirical studies: evolution and maintenance of dimorphic breeding systems. In: Geber MA, Dawson TE, Delph LE (eds) Gender and sexual dimorphism in flowering plants. Springer, New York, pp 61–95
Wu XQ, Li AM, Zhang DX (2009) Cryptic self-incompatibility and distyly in Hedyotis acutangula Champ. (Rubiaceae). Plant Biol 12(3):484–494
Acknowledgments
This work was supported by the National Natural Science Fundation of China (grant nos. 31170184, 31070161 and 31000109) and by the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-Z-6-6 and KZCX2-YW-414).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Luo, Z., Wu, X. et al. Functional dioecy in Morinda parvifolia (Rubiaceae), a species with stigma-height dimorphism. Plant Syst Evol 298, 775–785 (2012). https://doi.org/10.1007/s00606-011-0588-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-011-0588-y