Abstract
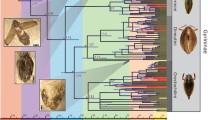
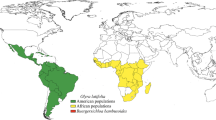
Nearly all of the species diversity in Piperaceae is encompassed within Piper and Peperomia. Both genera are pan-tropical with areas of diversification in the Neotropics and Southeast Asia. Piperaceae are less diverse in Africa with only two native species of Piper. This study examines the distribution of both Piper and Peperomia with representative samples from the Neotropics, Asia, Pacific Islands, and Africa. Molecular dating is used to place an age for the crown clades of Piper and Peperomia as well as ages for diversification within the clades. Both genera have origins in the late Cretaceous, but species level diversification occurred much later in the Tertiary. Biogeography of both genera are correlated with paleoclimate evidence to better explain the distribution and diversification of these large genera.


Similar content being viewed by others
References
Alice LA, Campbell CS (1999) Phylogeny of Rubus (Rosaceae) based on nuclear ribosomal DNA internal transcribed spacer region sequences. Amer J Bot 86:81–97
Alsos IG, Eidesen PB, Ehrich D, Skrede I, Westergaard K, Jacobsen FH, Landvik JY, Taberlet P, Brochmann C (2007) Frequent long-distance plant colonization in the changing arctic. Science 316:1606–1609
Aris-Brosou S, Yang Z (2002) Effects of models of rate evolution on estimation of divergence dates with special reference to the metazoan 18S rRNA phylogeny. Syst Biol 51:703–714
Australia Plant Names Index. http://www.anbg.gov.au/cpbr/databases/apni.html. Last visited October 2007
Axelrod DI (1972) Edaphic aridity as a factor in angiosperm evolution. Amer Naturalist 106:311–320
Axelrod DI, Raven PH (1978) Late Cretaceous and Tertiary vegetation history of Africa. In: Werger MJA (ed) Biogeography and ecology of southern Africa. Dr W. Junk bv Publishers, The Hague, pp 77–130
Baldwin BG, Robichaux RH (1995) Historical biogeography and ecology of the Hawaiian silversword alliance (Asteraceae): new molecular phylogenetic perspectives. In: Wagner WL, Funk VA (eds) Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington, DC, pp 259–189
Baldwin BG, Kyhos DW, Dvorak J (1990) Chloroplast DNA variation and adaptive radiation in the Hawaiian silversword alliance (Asteraceae- Madiinae). Ann Missouri Bot Gard 77:96–109
Baldwin BG, Kyhos DW, Dvorak J, Carr GD (1991) Chloroplast DNA evidence for a North American origin of the Hawaiian silversword alliance (Asteraceae). Proc Natl Acad Sci USA 88:1840–1843
Behrensmeyer AK, Damuth J, DiMichele W, Potts R, Sues HD, Wing S (1992) Terrestrial ecosystems through time. University of Chicago Press, Chicago
Bell CD (2007) Phylogenetic placement and biogeography of the North American species of Valerianella (Valerianaceae: Dipsacales) based on chloroplast and nuclear DNA. Molec Phylogenet Evol 44:929–941
Bell CD, Donoghue MJ (2005) Phylogeny and biogeography of Valerianaceae (Dipsacales) with special reference to the South American valerians. Organisms. Divers Evol 5:147–159
Borsch T, Hilu KW, Quandt D, Wilde V, Neinhuis C, Barthlott W (2003) Noncoding plastid trnT-trnF sequences reveal a well resolved phylogeny of basal angiosperms. J Evol Biol 16:558–576
Borsch T, Löhne C, Müller K, Wanke S, Worberg A, Barthlott W, Neinhus C, Hilu KW, Quandt D (2005) Towards understanding basal angiosperm diversification: recent insights using fast evolving genomic regions. Nova Acta Leopoldina 342:85–110
Carine MA, Russell SJ, Santos-Guerra A, Francisco-Ortega J (2004) Relationships of the Macaronesian and Mediterranean floras: molecular evidence for multiple colonizations into Macaronesia and back-colonization of the continent in Convolvulus (Convolvulaceae). Amer J Bot 91:1070–1085
Cheng Y-Q, Xia N-H, Gilbert MG (1999) Piperaceae. In: Wu Z-Y, Raven PH (eds) Flora of China, Cycadaceae through Fagaceae. Missouri Botanical Garden Press. St. Louis, Missouri, USA, pp 110–129
Chew W-L (1972) The genus Piper (Piperaceae) in New Guinea, Solomon Islands, and Australia, 1. J Arnold Arbor 53:1–25
Conti E, Eriksson T, Schönenberger J, Sytsma KJ, Baum DA (2002) Early Tertiary out-of-India dispersal of Crypteroniaceae: evidence from phylogeny and molecular dating. Evolution 56:1931–1942
Cox CB, Moore PD (2000) Biogeography. An ecological and evolutionary approach, 6th edn. Blackwell, London
Crisp MD, Cook LG (2007) A congruent molecular signature of vicariance across multiple plant lineages. Molec Phylogenet Evol 43:1106–1117
Cronk QCB, Kiehn M, Wagner WL, Smith JF (2005) Evolution of Cyrtandra (Gesneriaceae) in the Pacific Ocean: the origin of a supertramp clade. Amer J Bot 92:1017–1024
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London
Davis CC, Bell CD, Fritsch PW, Mathews S (2002) Phylogeny of Acridocarpus-Brachylon (Malpighiaceae): implications for Tertiary tropical floras and Afroasian biogeography. Evolution 56:2395–2405
Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ (2005) Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. Amer Naturalist 165:E36–E65
de Groot H, Wanke S, Neinhuis C (2006) Revision of the genus Aristolochia (Aristolochiaceae) in Africa, Madagascar and adjacent islands. Bot J Linn Soc 151:219–238
DeJoode DR, Wendel JF (1992) Genetic diversity and origin of the Hawaiian islands cotton, Gossypium tomentosum. Amer J Bot 79:1311–1319
de Queiroz A (2005) The resurrection of oceanic dispersal in historical biogeography. Trends Ecol Evol 20:68–73
Des Marais DL, Smith AR, Britton DM, Pryer KM (2003) Phylogenetic relationships and evolution of extant horsetails, Equisetum, based on chloroplast DNA sequence data (rbcL and trnL-F). Int J Pl Sci 164:737–751
Dick CW, Abdul-Salim K, Bermingham E (2003) Molecular systematic analysis reveals cryptic Tertiary diversification of a widespread tropical rain forest tree. Amer Naturalist 162:691–703
Doyle JA, Endress PK (2000) Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. Int J Pl Sci 161:S121–S151
Doyle JA, Endress PK (2007) Integrating early Cretaceous fossils into the phylogeny of recent angiosperms. Plant Biology and Botany 2007, p 150 (abstract)
Dutech C, Maggia L, Tardy C, Joly HI, Jarne P (2003) Tracking a genetic signal of extinction-recolonization events in a neotropical tree species: Vouacapoua americana Aublet in French Guiana. Evolution 57:2753–2764
Drummond A. J., Rambaut A. (2005) BEAST v 1.3. http://evolve.zoo.ox.ac.uk/beast/
Eklund H, Doyle JA, Herendeen PS (2004) Morphological phylogenetic analysis of living and fossil Chloranthaceae. Int J Pl Sci 165:107–151
Ellstrand NC, Schierenbeck KA (2000) Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci USA 97:7043–7050
Farris SJ, Källersjö M, Kluge AG, Bult C (1994) Testing significance of incongruence. Cladistics 10:315–319
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fleming T (1988) The short-tailed fruit bat: a study in plant-animal interactions. University of Chicago Press, Chicago
Fleming T (2004) Dispersal ecology of Neotropical Piper shrubs and treelets. In: Dyer LA, Palmer ADN (eds) Piper: A model genus for studies of Phytochemistry, Ecology, and Evolution. Kluwer, New York, pp 58–77
Friis EM, Pedersen KR, Crane PR (1995) Appomattoxia ancistrophora gen. et sp. nov., a new early Cretaceous plant with similarities to Circaeaster and extant Magnoliidae. Amer J Bot 82:933–943
Ganders FR, Berbee M, Pirseyedi M (2000) ITS based sequence phylogeny in Bidens (Asteraceae): evidence for the continental relatives of Hawaiian and Marquesan Bidens. Syst Bot 25:122–133
Gentry AH (1982) Neotropical floristic diversity: phytogeographical connections between Central and South America, Pleistocene climatic fluctuations, or an accident of the Andean orogeny? Ann Missouri Bot Gard 69:557–593
Gentry AH (1989) Speciation in tropical forests. In: Holm-Nielsen LB, Nielsen IC, Balslev H (eds) Tropical forests: botanical dynamics, speciation and diversity. Academic Press, London, pp 113–134
Givnish TJ, Sytsma KJ, Hahn WJ, Smith JF (1995) Molecular evolution, adaptive radiation, and geographic speciation in Cyanea (Campanulaceae, Lobelioideae). In: Wagner WL, Funk VA (eds) Hawaiian biogeography: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington, DC, pp 288–337
Givnish TJ, Knox E, Patterson TB, Hapeman JR, Palmer JD, Sytsma KJ (1996) The Hawaiian lobelioids are monophyletic and underwent a rapid initial radiation roughly 15 million years ago. Amer J Bot 83:159 (abstract)
Givnish TJ, Evans TM, Zjhra ML, Patterson TB, Berry PE, Sytsma KJ (2000) Molecular evolution, adaptive radiation, and geographic diversification in the amphiatlantic family Rapateaceae: evidence from ndhF sequences and morphology. Evolution 54:1915–1937
Givnish TJ, Millam KC, Evans TM, Hall JC, Pires JC, Berry PE, Sytsma KJ (2004) Ancient vicariance or recent long-distance dispersal? Inferences about phylogeny and South American-African disjunctions in Rapateaceae and Bromeliaceae based on ndhF sequence data. Int J Pl Sci 165:S35–S54
Goldman N, Anderson JP, Rodrigo AG (2000) Likelihood-based tests in phylogenetics. Syst Biol 49:652–670
Gorchov DL, Cornejo F, Ascorra CF, Jaramillo M (1993) The role of seed dispersal in the natural regeneration of rain forest after strip-cutting in the Peruvian Amazon. Pl Ecol 107–108:339–349
Gorchov DL, Cornejo F, Ascorra CF, Jaramillo M (1995) Dietary overlap between frugivorous birds and bats in the Peruvian Amazon. Oikos 74:235–250
Gregory-Wodzicki KM (2000) Uplift history of the central and northern Andes: a review. GSA Bull 112:1091–1105
Grieg N (2004) Introduction. In: Dyer LA, Palmer ADN (eds) Piper: A model genus for studies of Phytochemistry, Ecology, and Evolution. Kluwer, New York, pp 1–4
Hall R (1998) The plate tectonics of Cenozoic Southeast Asia and the distribution of land and sea. In: Hall R, Holloway JD (eds) Biogeography and geological evolution of Southeast Asia. Backhuys Publishers, Leiden, The Netherlands, pp 99–131
Hasebe M, Ando T, Iwatsuki K (1998) Intrageneric relationships of maple trees based on the chloroplast DNA restriction fragment length polymorphisms. J Pl Res 111:441–451
Helfgott DM, Francisco-Ortega J, Santos-Guerra A, Jansen RK, Simpson BB (2000) Biogeography and breeding system evolution of the woody Bencomia alliance (Rosaceae) in Macaronesia based on ITS sequence data. Syst Bot 25:82–97
Howarth DG, Gardner DE, Morden CW (1997) Phylogeny of Rubus subgenus Ideobatus (Rosaceae) and its implications toward colonization of the Hawaiian islands. Syst Bot 22:433–441
Howarth DG, Gustafsson MHG, Baum DA, Motley TJ (2003) Phylogenetics of the genus Scaevola (Goodeniaceae): implication for dispersal patterns across the Pacific Basin and colonization of the Hawaiian islands. Amer J Bot 90:915–923
Horn SP, Sanford RL, Dilcher D, Lott TA, Renne PR, Wiemann MC, Cozadd D, Vargas O (2003) Pleistocene plant fossils in and near La Selva Biological Station, Costa Rica. Biotropica 35:434–441
Huang J, Price RA (2003) Estimation of the age of extant Ephedra using chloroplast rbcL sequence data. Molec Biol Evol 20:435–440
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Hughes C, Eastwood R (2006) Island radiations on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proc Natl Acad Sci USA 103:10334–10339
Humphries CJ, Parenti LR (1999) Cladistic biogeography. Oxford University Press, Oxford, UK
Ickert-Bond SM, Wojciechowski MF (2004) Phylogenetic relationships in Ephedra (Gnetales): evidence from nuclear and chloroplast DNA sequence data. Syst Bot 29:834–849
Jaramillo C, Rueda MJ, Mora G (2006) Cenozoic plant diversity in the Neotropics. Science 311:1893–1896
Jaramillo MA, Manos PS (2001) Phylogeny and patterns of floral diversity in the genus Piper (Piperaceae). Amer J Bot 88:706–716
Jaramillo MA, Marquis R (2004) Future research in Piper biology. In: Dyer LA, Palmer ADN (eds) Piper: A model genus for studies of Phytochemistry, Ecology, and Evolution. Kluwer, New York, pp 199–203
Jaramillo MA, Kramer EM (2007) Molecular evolution of the petal and stamen identity genes, APETALA3 and PISTILLATA, after petal loss in the Piperales. Molec Phylogenet Evol 44:598–609
Jaramillo MA, Manos PS, Zimmer EA (2004) Phylogenetic relationships of the perianthless Piperales: reconstructing the evolution of floral development. Int J Pl Sci 165:403–416
Jaramillo MA, Callejas R, Davidson C, Smith JF, Stevens AC, Tepe EJ (in press) A phylogeny of the tropical genus Piper (Piperaceae) using ITS and the chloroplast intron psbJ-petA. Syst Bot
Johnson KR, Ellis B (2002) A tropical rainforest in Colorado 1.4 million years after the Cretaceous-Tertiary boundary. Science 296:2379–2383
Kim Y-D, Jansen RK (1998) Chloroplast DNA restriction site variation and phylogeny of the Berberidaceae. Amer J Bot 85:1766–1778
Kim Y-D, Kim S-H (1999) Phylogeny of Weigela and Diervilla (Caprifoliaceae) based on nuclear rDNA ITS sequences: biogeographic and taxonomic implications. J Pl Res 112:331–341
Kishino H, Hasegawa M (1989) Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in Hominoidea. J Molec Evol 29:170–179
Kishino H, Thorne JL, Bruno WJ (2001) Performance of a divergence time estimation method under a probabilistic model of rate evolution. Molec Biol Evol 18:352–361
Knox EB, Downie SR, Palmer JD (1993) Chloroplast genome rearrangements and the evolution of giant lobelias from herbaceous ancestors. Molec Biol Evol 10:414–430
Kubitzki K, Krutzsch W (1996) Origins of East and South Asian plant diversity. In: Auluo Z, Sugong W (eds) Floristic characteristics and diversity of East Asian plants. Springer, Berlin, pp 65–70
Lamb S (2004) Devil in the mountain: a search for the origin of the Andes. Princeton University Press, Princeton, NJ
Lindqvist C, Albert VA (2002) Origin of the Hawaiian endemic mints within North American Stachys (Lamiaceae). Amer J Bot 89:1709–1724
Lee NS, Sang T, Crawford DJ, Yeau SH, Kim S-C (1996) Molecular divergence between disjunct taxa in eastern Asia and eastern North America. Amer J Bot 83:1373–1378
Mack RN, Simberloff D, Lonsdale WM (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
MacPhail MK, Partridge AD, Truswell EM (1999) Fossil pollen records of the problematical primitive angiosperm family Lactoridaceae in Australia. Pl Syst Evol 214:199–210
Magallón S, Sanderson MJ (2001) Absolute diversification rates in angiosperm clades. Evolution 55:1762–1780
Mai DH (1995) Tertiäre Vegetationsgeschichte Europas. Fischer, Jena
Manchester SR (1989) Early history of the Juglandaceae. Pl Syst Evol 162:231–250
Manchester SR (1991) Cruciptera, a new Juglandaceous winged fruit from the Eocene and Oligocene of western North America. Syst Bot 16:715–725
Manchester SR (1999) Biogeographical relationships of North American Tertiary floras. Ann Missouri Bot Garden 86:472–522
Manchester SR, Wheeler EA (1993) Extinct juglandaceous wood from the Eocene of Oregon and its implications for xylem evolution in the Juglandaceae. IAWA J 14:103–111
Marquis RJ (2004) Biogeography of Neotropical Piper. In: Dyer LA, Palmer ADN (eds) Piper: A model genus for studies of Phytochemistry, Ecology, and Evolution. Kluwer, New York, pp 78–96
McGlone MS (2005) Goodbye Gondwana. J Biogeography 32:739–740
Miquel F. A. (1843–1844) Systema Piperacearum. H. A. Kramer, Rotterdam.
Möller M, Cronk QCB (2001) Phylogenetic studies in Streptocarpus (Gesneriaceae): reconstruction of biogeographic history and distribution patterns. Syst Geogr Plants 71:545–555
Morat P (1993) Our knowledge of the flora of New Caledonia: endemism and diversity in relation to vegetation types and substrates. Biodivers Lett 1:72–81
Morley RJ (2000) Origin and evolution of tropical rain forests. Wiley, New York
Morris AB, Bell CD, Clayton JW, Judd WS, Soltis DE, Soltis PS (2007) Phylogeny and divergence time estimation in Illicium with implications for New World biogeography. Syst Bot 32:236–249
Nelson G, Platnick NI (1981) Systematics and biogeography: cladistics and vicariance. Columbia University Press, New York
Neinhuis C, Wanke S, Hilu KW, Müller K, Borsch T (2005) Phylogeny of Aristolochiaceae based on parsimony, likelihood, and Bayesian analyses of trnL-trnF sequences. Pl Syst Evol 250:7–26
Nepokroeff M, Sytsma KJ, Wagner WL, Zimmer EA (2003) Reconstructing ancestral patterns of colonization and dispersal in the Hawaiian understory tree genus Psychotria (Rubiaceae): a comparison of parsimony and likelihood approaches. Syst Biol 52:820–838
Nickrent D, L, Blarer A, Qiu Y-L, Soltis DE, Soltis PS, Zanis M (2002) Molecular data place Hydnoraceae with Aristolochiaceae. Amer J Bot 89:1809–1817
Olmstead RG, Palmer JD (1994) Chloroplast DNA systematics: a review of methods and data analysis. Amer J Bot 81:1205–1224
Pennington RT, Lavin M, Prado DE, Pendry CA, Pell SK, Butterworth CA (2004) Historical climate change and speciation: neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Phil Trans Royal Soc London. Series B 359:515–537
Petit RJ, Bialozyt R, Garnier-Géré P, Hampe A (2004) Ecology and genetics of tree invasions: from recent introductions to Quaternary migrations. Forest Ecol Management 197:117–137
Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: Advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol 53:793–808
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Poux C, Madsen O, Marquard E, Vieites DR, Jong WWD, Vences M (2005) Asynchronous colonization of Madagascar by the four endemic clades of primates, tenrecs, carnivores, and rodents as inferred from nuclear genes. Syst Biol 54:719–730
Prance GT (1982) Biological diversification in the tropics. Columbia University Press, New York
Qiu Y-L, Parks CR, Chase MW (1995) Molecular divergence in eastern Asian-eastern North American disjunct section Rytidospermum of Magnolia (Magnoliaceae). Amer J Bot 82:1589–1598
Qiu Y-L, Lee J, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Savolainen V, Chase MW (1999) The earliest angiosperms: evidence from mitochondrial, plastid, and nuclear genomes. Nature 402:404–407
Quijano-Abril MA, Callejas-Posada R, Miranda-Esquivel DR (2006) Areas of endemism and distribution patterns for Neotropical Piper species (Piperaceae). J Biogeogr 33:1266–1278
Rambaut A, Bromham L (1998) Estimating divergence dates from molecular sequences. Molec Biol Evol 15:442–448
Raven PH, Axelrod DI (1974) Angiosperm biogeography and past continental movements. Ann Missouri Bot Gard 61:539–673
Ree RH, Moore BR, Webb CO, Donoghue MJ (2005) A likelihood framework for inferring the evolution of geographic range on phylogenetic trees. Evolution 59:2299–2311
Reeves G, Chase MW, Goldblatt P, Fay MF, Cox AV, LeJeune B, Souza-Chies T (2001) Molecular systematics of Iridaceae: evidence from four plastid DNA regions. Amer J Bot 88:2074–2087
Renner SS (2004) Plant dispersal across the tropical Atlantic by wind and sea currents. Int J Pl Sci 165:S23–S33
Renner SS, Clausing G, Meyer K (2001) Historical biogeography of Melastomataceae: the roles of Tertiary migration and long-distance dispersal. Amer J Bot 88:1290–1300
Richardson JE, Pennington RT, Pennington TD, Hollingsworth PM (2001) Rapid diversification of a species-rich genus of Neotropical rain forest trees. Science 293:2242–2245
Ridley H (1930) The dispersal of plants throughout the world. Ashford, Reeve
Ritz CM, Martins L, Mecklenberg R, Goremykin V, Hellwig FH (2007) The molecular phylogeny of Rebutia (Cactaceae) and its allies demonstrates the influence of paleogeography on the evolution of South American mountain cacti. Amer J Bot 94:1321–1332
Ronquist F. (1996) DIVA. Ver. 1.1 Computer program and manual. Available by anonymous FTP (ftp.sysbot.uu.se) from Uppsala University, Uppsala, Sweden
Ronquist F (1997) Dispersal-vicariance analysis: a new approach to the quantification of historical biogeography. Syst Biol 45:195–203
Sanderson MJ (1997) A nonparametric approach to estimating divergence times in the absence of rate constancy. Molec Biol Evol 14:1218–1231
Sanderson MJ (2002) Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Molec Biol Evol 19:101–109
Sanderson MJ (2003) r8s: inferring absolute rates of molecular evolution and divergence times in the absence of a molecular clock. Bioinformatics 19:301–302
Sanmartín I, Ronquist F (2004) Southern hemisphere biogeography inferred by event-based models: plant versus animal patterns. Syst Biol 53:216–243
Särkinen TE, Newman MF, Maas PJM, Maas H, Poulsen AD, Harris DJ, Richardson JE, Clark A, Hollingsworth M, Pennington RT (2007) Recent oceanic long-distance dispersal and divergence in the amphi-Atlantic rain forest genus Renealmia L. f. (Zingiberaceae). Molec Phylogenet Evol 44:968–980
Schnabel A, Wendel JF (1998) Cladistic biogeography of Gleditsia (Leguminosae) based on ndhF and rpl16 chloroplast gene sequences. Amer J Bot 85:1753–1765
Seelanen T, Schnabel A, Wendel JF (1997) Congruence and consensus in the cotton tribe (Malvaceae). Syst Bot 22:259–290
Shimodaira H, Hasegawa M (1999) Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Molec Biol Evol 16:1114–1116
Simmons MP, Ochoterena H (2000) Gaps as characters in sequence-based phylogenetic analyses. Syst Biol 49:369–381
Smith SA (1975) The genus Macropiper (Piperaceae). Biol J Linn Soc 71:1–38
Smith S. A. (2006) AReA: Ancestral range analysis v. 2.1. http://blackrim.org/programs/area.html
Smith SY, Stockey RA (2007) Establishing a fossil record for the perianthless Piperales: Saururus tuckerae sp. nov. (Saururaceae) from the Middle Eocene Princeton Chert. Amer J Bot 94:1642–1657
Smith JF, Funke MM, Woo VL (2006) A duplication of gcyc predates divergence within tribe Coronanthereae (Gesneriaceae): phylogenetic analysis and evolution. Pl Syst Evol 261:245–256
Smith JF, Hileman LC, Powell M, Baum DA (2004) Evolution of gcyc, a Gesneriaceae homolog of CYCLOIDEA, within subfamily Gesnerioideae (Gesneriaceae). Molec Phylogenet Evol 31:765–779
Soltis DE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, Savolainen V, Hahn WH, Hoot SB, Fay MF, Axtell M, Swensen SM, Prince LM, Kress WJ, Nixon KC, Farris JS (2000) Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot J Linn Soc 133:381–461
Strand AE, Leebens-Mack J, Milligan BG (1997) Nuclear DNA-based markers for plant evolutionary biology. Molec Ecol 6:113–118
Stuessy TF, Crawford DJ, Anderson GJ, Jensen RJ (1998a) Systematics, biogeography and conservation of Lactoridaceae Pers. Pl Ecol Evol Syst 1:267–290
Stuessy TF, Crawford DJ, Marticorena C, Rodriguez R (1998b) Island biogeography of angiosperms of the Juan Fernandez archipelago. In: Stuessy TF, Ono M (eds) Evolution and speciation of island plants. Cambridge University Press, Cambridge UK, pp 121–138
Swenson U, Bremer K (1997) Pacific biogeography of the Asteraceae genus Abrotanella (Senecioneae, Blennospermatinae). Syst Bot 22:493–508
Swenson U, Hill RS, McLoughlin S (2001) Biogeography of Nothofagus supports the sequence of Gondwana break-up. Taxon 50:1025–1041
Swofford D. L. (2002) PAUP* Phylogenetic analysis using parsimony (* and Other Methods). Version 10. Sinauer Associates, Sunderland, Massachusetts
Thorne JL, Kishino H (2002) Divergence time estimation and rate evolution with multilocus data sets. Syst Biol 51:689–702
Thorne JL, Kishino H, Painter IS (1998) Estimating the rate of evolution of the rate of molecular evolution. Molec Biol Evol 15:1647–1657
Tiffney BH (1984) Seed size, dispersal syndromes, and the rise of the angiosperms: evidence and hypothesis. Ann Missouri Bot Gard 71:551–576
Tiffney BH (1985a) Perspectives on the origin of the floristic similarity between eastern Asia and eastern North America. J Arnold Arbor 66:73–94
Tiffney BH (1985b) The Eocene North Atlantic land bridge: its importance in Tertiary and modern phytogeography of the Northern hemisphere. J Arnold Arbor 66:243–273
Tiffney BH, Manchester SR (2001) The use of geological and paleontological evidence in evaluating plant phylogenetic hypotheses in the Northern hemisphere Tertiary. Int J Pl Sci 162:S3–S17
Upchurch GR, Wolfe JA (1987) Mid-Cretaceous to early Tertiary vegetation and climate: evidence from fossil leaves and wood. In: Friis EM, Chaloner WG, Crane PR (eds) The origins of angiosperms and their biological consequences. Cambridge University Press, Cambridge, UK, pp 75–105
Upchurch GR, Wolfe JA (1993) Cretaceous vegetation of the western interior and adjacent regions of North America. In: Kauffman EG, Caldwell WGE (eds) Cretaceous evolution of the western interior basin. Geological Association of Canada, Special Paper, vol 39, pp 243–281
Valdebenito HA, Stuessy TF, Crawford DJ (1990) A new biogeographic connection between islands in the Atlantic and Pacific oceans. Nature 347:549–550
Valdebenito HA, Stuessy TF, Crawford DJ, Silva OM (1992) Evolution of Peperomia (Piperaceae) in the Juan Fernandez Islands, Chile. Pl Syst Evol 182:107–119
Wagner WL, Funk VA (1995) Hawaiian biogoegraphy: evolution on a hot spot archipelago. Smithsonian Institution Press, Washington, DC
Wagner W. L., Herbst D. R., Sohmer S. H. (1990) Manual of the flowering plants of Hawai’i. University of Hawai’i Press, Honolulu, Hawai’i, USA
Wanke S, Samain M-S, Vanderschaeve L, Mathieu G, Goetghebeur P, Neinhuis C (2006) Phylogeny of the genus Peperomia (Piperaceae) inferred from the trnK/matK region (cpDNA). Pl Biol 8:93–102
Wanke S, Jaramillo MA, Borsch T, Samain M-S, Quandt D, Neinhuis C (2007a) Evolution of the Piperales - matK and trnK intron sequence data reveal lineage specific resolution contrast. Molec Phylogenet Evol 42:477–497
Wanke S, Vanderschaeve L, Mathieu G, Neinhuis C, Goetghebeur P, Samain M-S (2007b) From forgotten taxon to a missing link? The position of the genus Verhuellia (Piperaceae) revealed by molecules. Ann Bot 99:1231–1238
Wen J, Stuessy TF (1993) The phylogeny and biogeography of Nyssa (Cornaceae). Syst Bot 18:68–79
Wen J, Jansen RK (1995) Morphological and molecular comparisons of Campsis grandiflora and C. radicans (Bignoniaceae), an eastern Asian and eastern North American vicariad species pair. Pl Syst Evol 196:173–183
Wen J, Zimmer EA (1996) Phylogeny and biogeography of Panax L. (the ginseng genus, Araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Molec Phylogenet Evol 6:167–177
Wen J, Janzen RK, Zimmer EA (1996) Phylogenetic relationships and DNA sequence divergence of eastern Asian and eastern North American disjunct plants. In: Nei M, Takahata N (eds) Current topics of molecular evolution. Jointly published by the Pennsylvania State University and the Graduate University for Advanced Studies, Hayama, Japan, pp 37–44
Wen J, Shi S, Jansen RK, Zimmer EA (1998) Phylogeny and biogeography of Aralia sect. Aralia (Araliaceae). Amer J Bot 85:866–875
Whitmore TC (1998) An introduction to tropical rain forests, 2nd edn. Oxford University Press, Oxford
Whitmore TC, Prance GT (1987) Biogoegraphy and Quaternary history in tropical America. Oxford University Press, New York
Williams DA, Overholt WA, Cuda JP, Hughes CR (2005) Chloroplast and microsatellite DNA diversities reveal the introduction history of Brazilian peppertree (Schinus terebinthifolius) in Florida. Molec Ecol 14:3643–3656
Wing SL, Boucher LD (1998) Ecological aspects of the Cretaceous flowering plant radiation. Annual Rev Earth Planet Sci 26:379–421
Winkworth RC, Wagstaff SJ, Glenny D, Lockhart PJ (2005) Evolution of the New Zealand mountain flora: origins, diversification and dispersal. Organisms Div Evol 5:237–247
Wolfe JA, Upchurch GR (1987) North American nonmarine climates and vegetation during the Late Cretaceous. Palaeog Palaeoclim Palaeoecol 61:33–77
Won H, Renner SS (2006) Dating dispersal and radiation in the Gymnosperm Gnetum (Gnetales) - Clock calibration when outgroup relationships are uncertain. Syst Biol 55:610–622
Wright SD, Yong CG, Dawson JW, Whittaker DJ, Gardner RC (2000) Riding the ice age El Niño? Pacific biogeography and evolution of Metrosideros (Myrtaceae) inferred from nuclear ribosomal DNA. Proc Natl Acad Sci USA 97:4118–4123
Wright SD, Yong CG, Dawson JW, Whittaker DJ, Gardner RC (2001) Stepping stones to Hawaii: a trans-equatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS plus ETS). J Biogeogr 28:769–774
Xiang Q-Y, Crawford DJ, Wolfe AD, Tang Y-C, DePamphilis CE (1998) Origin and biogeography of Aesculus (Hippocastanaceae): a molecular phylogenetic perspective. Evolution 52:988–997
Yoder AD, Irwin JA, Payseur BA (2001) Failure of the ILD to determine data combinability for slow loris phylogeny. Syst Biol 50:408–424
Yoo MJ, Bell CD, Soltis PS, Soltis DE (2005) Divergence times and historical biogeography of Nymphaeales. Syst Bot 30:693–704
Young KR, Ulloa UC, Luteyn JL, Knapp S (2002) Plant evolution and endemism in Andean South America: an introduction. Bot Rev 68:4–21
Zanis MJ, Soltis DE, Soltis PS, Mathews S, Donoghue MJ (2002) The root of the angiosperms revisited. Proc Natl Acad Sci USA 99:6848–6853
Zavada MS, Benson JM (1987) First fossil evidence for the primitive angiosperm family Lactoridaceae. Amer J Bot 74:1590–1594
Zhang L-B, Renner SS (2003) The deepest splits in Chloranthaceae as resolved by chloroplast sequences. Int J Pl Sci 164:S383–S392
Zhang L-B, Simmons MP, Renner SS (2007) A phylogeny of Anisophyllaceae based on six nuclear and plastic loci: ancient disjunctions and recent dispersal between South America, Africa, and Asia. Molec Phylogenet Evol 44:1057–1067
Zhou S, Renner SS, Wen J (2006) Molecular phylogeny and inter- and intracontinental biogeography of Calycanthaceae. Molec Phylogenet Evol 39:1–15
Ziegler AM, Eshel G, Rees PM, Rothfus TA, Rowley DB (2003) Tracing the tropics across land and sea: Permian to present. Lethaia 36:227–254
Acknowledgments
We would like to acknowledge that Chris Fouminyam of the Limbe Botanical Garden, Limbe, Cameroon; George Owusu Afriye of the Aburri Botanical Garden, Aburri, Ghana; Jardins et Conservatoire Botanique de Nancy, France; Tim Flynn and Dave Lorence of the National Tropical Botanical Garden, Lawai, Hawaii; C. W. Morden of the University of Hawaii; Allan Bornstein of Southeast Missouri State University; Amit Jain, Mindie Funke, Bree Draper, and Wee Seng Wong of Boise State University; Audrey Mollerup of Whitman College; The Missouri Botanical Garden; Li Jia-Mei and Wang Yin-Zheng of the Chinese Academy of Sciences all contributed or assisted in obtaining material and data for this project. We would also like to thank Stefan Wanke and Marie-Stephanie Samain for their comments and for correcting our understanding of Peperomia distribution patterns and two anonymous reviewers who made important suggestions that improved the manuscript. The use of the Beowulf Computer Cluster at Boise State University facilitated our data analysis. Funding for this project was provided by NSF grant DEB-0107763 including RUI support, a Boise State University Faculty Research grant, and a grant from the Marjorie Moore Davidson Foundation, all to JFS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, J.F., Stevens, A.C., Tepe, E.J. et al. Placing the origin of two species-rich genera in the late cretaceous with later species divergence in the tertiary: a phylogenetic, biogeographic and molecular dating analysis of Piper and Peperomia (Piperaceae). Plant Syst Evol 275, 9–30 (2008). https://doi.org/10.1007/s00606-008-0056-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0056-5