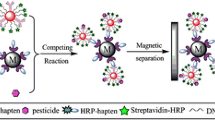
Abstract
A rapid detection method is introduced for residual trace levels of triazophos in water and agricultural products using an immunoassay based on catalytic hairpin self-assembly (CHA). The gold nanoparticle (AuNPs) surface was modified with triazophos antibody and sulfhydryl bio-barcode, and an immune competition reaction system was established between triazophos and its ovalbumin-hapten (OVA-hapten). The bio-barcode served as a catalyst to continuously induce the CHA reaction to achieve the dual signal amplification. The method does not rely on the participation of enzymes, and the addition of fluorescent materials in the last step avoids interfering factors, such as a fluorescence burst. The emitted fluorescence was detected at 489/521 nm excitation/emission wavelengths. The detection range of the developed method was 0.01–50 ng/mL for triazophos, and the limit of detection (LOD) was 0.0048 ng/mL. The developed method correlates well with the results obtained by LC–MS/MS, with satisfactory recovery and sensitivity. In sum, the designed method is reliable and provides a new approach to detect pesticide residues rapidly and quantitatively.
Graphical Abstract




modified by nanogold using colorimetric and UV spectrophotometric methods



Similar content being viewed by others
Change history
24 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00604-022-05325-2
References
Bhandari G, Zomer P, Atreya K, Mol HGJ, Yang X, Geissen V (2019) Pesticide residues in Nepalese vegetables and potential health risks. Environ Res 172:511–521. https://doi.org/10.1016/j.envres.2019.03.002
Agüera A, Contreras M, Fernandez-Alba AR (1993) Gas chromatographic analysis of organophosphorus pesticides of horticultural concern. J Chromatogr A 655(2):293–300. https://doi.org/10.1016/0021-9673(93)83235-K
Arias PG, Martínez-Pérez-Cejuela H, Combès A, Pichon V, Pereira E, Herrero-Martínez JM, Bravo M (2020) Selective solid-phase extraction of organophosphorus pesticides and their oxon-derivatives from water samples using molecularly imprinted polymer followed by high-performance liquid chromatography with UV detection. J Chromatogr A 1626:461346. https://doi.org/10.1016/j.chroma.2020.461346
Harischandra NR, Pallavi MS, Bheemanna M, PavanKumar K, Chandra Sekhara Reddy V, Udaykumar NR, Paramasivam M, Yadav S (2021) Simultaneous determination of 79 pesticides in pigeonpea grains using GC–MS/MS and LC–MS/MS. Food Chem 347:128986. https://doi.org/10.1016/j.foodchem.2020.128986
Acosta-Dacal A, Rial-Berriel C, Díaz-Díaz R, Bernal-Suárez MdM, Luzardo OP (2021) Optimization and validation of a QuEChERS-based method for the simultaneous environmental monitoring of 218 pesticide residues in clay loam soil. Sci Total Environ 753:142015. https://doi.org/10.1016/j.scitotenv.2020.142015
Noh HH, Kim CJ, Kwon H, Kim D, Moon BC, Baek S, Oh MS, Kyung KS (2020) Optimized residue analysis method for broflanilide and its metabolites in agricultural produce using the QuEChERS method and LC-MS/MS. Plos One 15(10):e0235526. https://doi.org/10.1371/journal.pone.0235526
Pogačnik L, Franko M (2001) Optimisation of FIA system for detection of organophosphorus and carbamate pesticides based on cholinesterase inhibition. Talanta 54(4):631–641. https://doi.org/10.1016/S0039-9140(01)00314-9
Xu Z, Dong J, Yang J, Wang H, Jiang Y, Lei H, Shen Y, Sun Y (2012) Development of a sensitive time-resolved fluoroimmunoassay for organophosphorus pesticides in environmental water samples. Anal Methods 4(10):3484. https://doi.org/10.1039/c2ay25534k
Yu G, Wu W, Zhao Q, Wei X, Lu Q (2015) Efficient immobilization of acetylcholinesterase onto amino functionalized carbon nanotubes for the fabrication of high sensitive organophosphorus pesticides biosensors. Biosens Bioelectron 68:288–294. https://doi.org/10.1016/j.bios.2015.01.005
Karczmarczyk A, Baeumner AJ, Feller K-H (2017) Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim Acta 243:82–89. https://doi.org/10.1016/j.electacta.2017.05.046
Liu B, Gong H, Wang Y, Zhang X, Li P, Qiu Y, Wang L, Hua X, Guo Y, Wang M (2018) A gold immunochromatographic assay for simultaneous detection of parathion and triazophos in agricultural products. Anal Methods 10:422–428. https://doi.org/10.1039/C7AY02481A
Nam JM (2003) Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301(5641):1884–1886. https://doi.org/10.1126/science.1088755
Sedighi A, Krull UJ (2019) Enhanced immunoassay using a rotating paper platform for quantitative determination of low abundance protein biomarkers. Anal Chem 91(8):5371–5379. https://doi.org/10.1021/acs.analchem.9b00502
Wang Q, Su J, Xu J, Xiang Y, Yuan R, Chai Y (2012) Dual amplified, sensitive electrochemical detection of pathogenic sequences based on biobarcode labels and functional graphene modified electrode. Sens Actuators B 163(1):267–271. https://doi.org/10.1016/j.snb.2012.01.050
Broto M, Salvador JP, Galve R, Marco MP (2018) Biobarcode assay for the oral anticoagulant acenocoumarol. Talanta 178:308–314. https://doi.org/10.1016/j.talanta.2017.09.006
Guan N, Li Y, Yang H, Hu P, Lu S, Ren H, Liu Z, Soo Park K, Zhou Y (2021) Dual-functionalized gold nanoparticles probe based bio-barcode immuno-PCR for the detection of glyphosate. Food Chem 338:128133. https://doi.org/10.1016/j.foodchem.2020.128133
Du Pengfei MJ, GeChen CZ, Jiang Z, Zhang Y, Zou P, Yongxin She FJ, Shao H, ShanshanWang LufeiZheng, JingWang, (2016) A competitive bio-barcode amplification immunoassay for small molecules based on nanoparticles. Sci Rep 6(1):38114. https://doi.org/10.1038/srep38114
Cui X, Jin M, Zhang C, Du P, Chen G, Qin G, Jiang Z, Zhang Y, Li M, Liao Y, Wang Y, Cao Z, Yan F, Abd El-Aty AM, Wang J (2019) Enhancing the sensitivity of the bio-barcode immunoassay for triazophos detection based on nanoparticles and droplet digital polymerase chain reaction. J Agric Food Chem 67(46):12936–12944. https://doi.org/10.1021/acs.jafc.9b05147
Du P, Jin M, Zhang C, Chen G, Cui X, Zhang Y, Zhang Y, Zou P, Jiang Z, Cao X, She Y, Jin F, Wang J (2018) Highly sensitive detection of triazophos pesticide using a novel bio-bar-code amplification competitive immunoassay in a micro well plate-based platform. Sens Actuators B 256:457–464. https://doi.org/10.1016/j.snb.2017.10.075
Zhang C, Jiang Z, Jin M, Du P, Chen G, Cui X, Zhang Y, Qin G, Yan F, Abd El-Aty AM, Hacimüftüoğlu A, Wang J (2020) Fluorescence immunoassay for multiplex detection of organophosphate pesticides in agro-products based on signal amplification of gold nanoparticles and oligonucleotides. Food Chem 326:126813. https://doi.org/10.1016/j.foodchem.2020.126813
Liu S, Wang Y, Ming J, Lin Y, Cheng C, Li F (2013) Enzyme-free and ultrasensitive electrochemical detection of nucleic acids by target catalyzed hairpin assembly followed with hybridization chain reaction. Biosens Bioelectron 49:472–477. https://doi.org/10.1016/j.bios.2013.05.037
Zhang M, Huo B, Yuan S, Ning B, Bai J, Peng Y, Liu B, Gao Z (2018) Ultrasensitive detection of T-2 toxin in food based on bio-barcode and rolling circle amplification. Anal Chim Acta 1043:98–106. https://doi.org/10.1016/j.aca.2018.09.007
Sukphattanaudomchoke C, Siripattanapipong S, Thita T, Leelayoova S, Piyaraj P, Mungthin M, Ruang-areerate T (2020) Simplified closed tube loop mediated isothermal amplification (LAMP) assay for visual diagnosis of Leishmania infection. Acta Trop 212:105651. https://doi.org/10.1016/j.actatropica.2020.105651
Cui Y, Fan S, Yuan Z, Song M, Hu J, Qian D, Zhen D, Li J, Zhu B (2021) Ultrasensitive electrochemical assay for microRNA-21 based on CRISPR/Cas13a-assisted catalytic hairpin assembly. Talanta 224:121878. https://doi.org/10.1016/j.talanta.2020.121878
Chen R, Sun Y, Huo B, Yuan S, Sun X, Zhang M, Yin N, Fan L, Yao W, Wang J, Han D, Li S, Peng Y, Bai J, Ning B, Liang J, Gao Z (2020) Highly sensitive detection of ochratoxin A based on bio-barcode immunoassay and catalytic hairpin assembly signal amplification. Talanta 208:120405. https://doi.org/10.1016/j.talanta.2019.120405
MOA, 2021. National food safety standard—maximum residue limits for pesticides in food. GB 2763–2021. Beijing, China.
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int 86(2):412–431. https://doi.org/10.1093/jaoac/86.2.412
Ma S, He J, Guo M, Sun X, Zheng M, Wang Y (2018) Ultrasensitive colorimetric detection of triazophos based on the aggregation of silver nanoparticles. Colloids Surf, A 538:343–349. https://doi.org/10.1016/j.colsurfa.2017.11.030
Ma Y, Zhao Y, Xu X, Ding S, Li Y (2021) Magnetic covalent organic framework immobilized gold nanoparticles with high-efficiency catalytic performance for chemiluminescent detection of pesticide triazophos. Talanta 235:122798. https://doi.org/10.1016/j.talanta.2021.122798
Wu M, Fan Y, Li J, Lu D, Guo Y, Xie L, Wu Y (2019) Vinyl phosphate-functionalized, magnetic, molecularly-imprinted polymeric microspheres’ enrichment and carbon dots’ fluorescence-detection of organophosphorus pesticide residues. Polymers 11(11):1770. https://doi.org/10.3390/polym11111770
Zhang C, Du P, Jiang Z, Jin M, Chen G, Cao X, Cui X, Zhang Y, Li R, Abd El-Aty AM, Wang J (2018) A simple and sensitive competitive bio-barcode immunoassay for triazophos based on multi-modified gold nanoparticles and fluorescent signal amplification. Anal Chim Acta 999:123–131. https://doi.org/10.1016/j.aca.2017.10.032
Funding
This study was financially supported by the National Key Research Program of China (No. 2019YFC1604503), NIEHS Superfund Research Program (No. P42 ES04699), Agricultural Science and Technology Innovation Program of CAAS (No. CAAS-ZDRW202011), and Ningbo Innovation Project for Agro-Products Quality and Safety (No. 2019CXGC007).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the original version of this article, the given name and family name of “AM Abd El-Aty” were incorrectly structured.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Abd El-Aty, A.M., Chen, G. et al. A competitive immunoassay for detecting triazophos based on fluorescent catalytic hairpin self-assembly. Microchim Acta 189, 114 (2022). https://doi.org/10.1007/s00604-022-05217-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05217-5