Abstract
Based on the laccase-mimicking activity of Cu2+-modified University of Oslo (UiO) metal–organic framework (UiO-67-Cu2+), we developed a colorimetric sensor array for distinguishing a series of phenols with different number and position of substituted hydroxyl group (-OH) and different substituent group on the benzene ring, including phenol, catechol, quinol, resorcinol, pyrogallol, phloroglucinol, o-chlorophenol, o-aminophenol, and o-nitrophenol. The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of phenolic compounds were obtained by theoretical calculation. The results show that the lower the LUMO energy level, the easier the chromogenic reaction occurs. The UiO-67-Cu2+-catalyzed phenol chromogenic reaction showed a good linearity in the range from 0.1 to 200 μM with limit of detection approximately 61 nM. Through the detection of phenol in tap water and river water, the recovery rate and RSD (n = 3) were calculated as 94.1~103% and 1.0~3.3, respectively, showing good recovery, reliable results, and outstanding stability. Therefore, the proposed colorimetric sensor array will have a great potential for the detection of phenols in the environment.
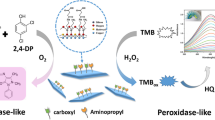
Graphical abstract

Schematic presentation of a simple and sensitive colorimetric strategy based on the laccase-mimicking activity of Cu2+-modified UiO-type metal–organic framework (MOFs, Uio-67-Cu2+) to distinguish phenols with analogous structures.






Similar content being viewed by others
References
Martinkova L, Kotik M, Markova E, Homolka L (2016) Biodegradation of phenolic compounds by Basidiomycota and its phenol oxidases: a review. Chemosphere 149:373–382. https://doi.org/10.1016/j.chemosphere.2016.01.022
Yang Y, Li X, Shen R, Liu Z, Ji D, Wang Y (2020) Seasonal variation and sources of derivatized phenols in atmospheric fine particulate matter in North China plain. J Environ Sci (China) 89:136–144. https://doi.org/10.1016/j.jes.2019.10.015
Torres E, Bustos-Jaimes I, Le Borgne S (2003) Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B Environ 46(1):1–15. https://doi.org/10.1016/s0926-3373(03)00228-5
Czaplicka M (2004) Sources and transformations of chlorophenols in the natural environment. Sci Total Environ 322(1–3):21–39. https://doi.org/10.1016/j.scitotenv.2003.09.015
Xu X, Wu S, Guo D, Niu X (2020) Construction of a recyclable oxidase-mimicking Fe3o4@Mnox-based colorimetric sensor array for quantifying and identifying chlorophenols. Anal Chim Acta 1107:203–212. https://doi.org/10.1016/j.aca.2020.02.024
Alkasir RS, Ganesana M, Won YH, Stanciu L, Andreescu S (2010) Enzyme functionalized nanoparticles for electrochemical biosensors: a comparative study with applications for the detection of bisphenol A. Biosens Bioelectron 26(1):43–49. https://doi.org/10.1016/j.bios.2010.05.001
Gao HY, Mao L, Li F, Xie LN, Huang CH, Shao J, Shao B, Kalyanaraman B, Zhu BZ (2017) Mechanism of Intrinsic chemiluminescence production from the degradation of persistent chlorinated phenols by the fenton system: a structure-activity relationship study and the critical role of quinoid and semiquinone radical intermediates. Environ Sci Technol 51(5):2934–2943. https://doi.org/10.1021/acs.est.6b04664
Kunitski M, Eicke N, Huber P, Kohler J, Zeller S, Voigtsberger J, Schlott N, Henrichs K, Sann H, Trinter F, Schmidt LPH, Kalinin A, Schoffler MS, Jahnke T, Lein M, Dorner R (2019) Double-slit photoelectron interference in strong-field ionization of the neon dimer. Nat Commun 10(1):1. https://doi.org/10.1038/s41467-018-07882-8
Garcia-Valverde MT, Lucena R, Cardenas S, Valcarcel M (2016) In-syringe dispersive micro-solid phase extraction using carbon fibres for the determination of chlorophenols in human urine by gas chromatography/mass spectrometry. J Chromatogr A 1464:42–49. https://doi.org/10.1016/j.chroma.2016.08.036
Arsad SS, Zainudin MAM, De Gobba C, Jongberg S, Larsen FH, Lametsch R, Andersen ML, Lund MN (2020) Quantitation of protein cysteine-phenol adducts in minced beef containing 4-methyl catechol. J Agric Food Chem 68(8):2506–2515. https://doi.org/10.1021/acs.jafc.9b07752
Zhang X, Yin J, Yoon J (2014) Recent advances in development of chiral fluorescent and colorimetric sensors. Chem Rev 114(9):4918–4959. https://doi.org/10.1021/cr400568b
Liu B, Zhuang J, Wei G (2020) Recent advances in the design of colorimetric sensors for environmental monitoring. Environ Sci: Nano 7(8):2195–2213. https://doi.org/10.1039/d0en00449a
Li MH, Chen JX, Wu WW, Fang YX, Dong SJ (2020) Oxidase-like Mof-818 nanozyme with high specificity for catalysis of catechol oxidation. J Am Chem Soc 142(36):15569–15574. https://doi.org/10.1021/jacs.0c07273
Wu S, Guo D, Xu X, Pan J, Niu X (2020) Colorimetric quantification and discrimination of phenolic pollutants based on peroxidase-like Fe3o4 nanoparticles. Sensors Actuators B Chem 303:127225–127233. https://doi.org/10.1016/j.snb.2019.127225
Alula MT, Madingwane ML (2020) Colorimetric quantification of chromium (Vi) ions based on oxidoreductase-like activity of Fe3o4. Sensors Actuators B Chem 324:128726–128736. https://doi.org/10.1016/j.snb.2020.128726
Liu M, Li J (2019) In-situ raman characterization of initial corrosion behavior of copper in neutral 3.5% (Wt.) Nacl solution. Materials (Basel) 12(13). https://doi.org/10.3390/ma12132164
Carniatoa GDS, Rocheta F, Rouleta H, Chaquinb P, Giessner-Prettreb C (1994) Theory assisted interpretation of copper phthalocyanine core levels Xps Spectra. J Electron Spectrosc Relat Phenom (67):189–209. https://doi.org/10.1016/0368-2048(93)02023-f
Koyappayil A, Kim HT, Lee MH (2021) ‘Laccase-like’ properties of coral-like silver citrate micro-structures for the degradation and determination of phenolic pollutants and adrenaline. J Hazard Mater 412:125211. https://doi.org/10.1016/j.jhazmat.2021.125211
Riva S (2006) Laccases: blue enzymes for green chemistry. Trends Biotechnol 24(5):219–226. https://doi.org/10.1016/j.tibtech.2006.03.006
Wang J, Huang R, Qi W, Su R, Binks BP, He Z (2019) Construction of a bioinspired laccase-mimicking nanozyme for the degradation and detection of phenolic pollutants. Appl Catal B Environ 254:452–462. https://doi.org/10.1016/j.apcatb.2019.05.012
An B, Zhang J, Cheng K, Ji P, Wang C, Lin W (2017) Confinement of ultrasmall Cu/Znox nanoparticles in metal-organic frameworks for selective methanol synthesis from catalytic hydrogenation of Co2. J Am Chem Soc 139(10):3834–3840. https://doi.org/10.1021/jacs.7b00058
Zhu L, Liu XQ, Jiang HL, Sun LB (2017) Metal-organic frameworks for heterogeneous basic catalysis. Chem Rev 117(12):8129–8176. https://doi.org/10.1021/acs.chemrev.7b00091
Huang Y, Ren J, Qu X (2019) Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev 119(6):4357–4412. https://doi.org/10.1021/acs.chemrev.8b00672
Hu J, Du W, Ji X, Yuan B, Liu Y, Guo M (2016) The chemistry, morphology, crystal structure and hydrophilicity properties of wood fibers treated by a magnetic immobilized laccase–mediator system. RSC Adv 6(39):32572–32579. https://doi.org/10.1039/c6ra00740f
Zalomaeva OV, Ivanchikova ID, Kholdeeva OA, Sorokin AB (2009) Kinetics and mechanism of the oxidation of alkyl substituted phenols and naphthols with Tbuooh in the presence of supported iron phthalocyanine. New J Chem 33(5).https://doi.org/10.1039/b821534k
Wang K, Feng D, Liu TF, Su J, Yuan S, Chen YP, Bosch M, Zou X, Zhou HC (2014) A series of highly stable mesoporous metalloporphyrin Fe-Mofs. J Am Chem Soc 136(40):13983–13986. https://doi.org/10.1021/ja507269n
Liang H, Lin F, Zhang Z, Liu B, Jiang S, Yuan Q, Liu J (2017) Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl Mater Interfaces 9(2):1352–1360. https://doi.org/10.1021/acsami.6b15124
Yoshikai N, Nakamura E (2012) Mechanisms of nucleophilic organocopper(I) reactions. Chem Rev 112(4):2339–2372. https://doi.org/10.1021/cr200241f
Glendening ED, Landis CR, Weinhold F (2011) Natural bond orbital methods. WIREs Comput Mol Sci 2(1):1–42. https://doi.org/10.1002/wcms.51
Filatov M, Lee S, Nakata H, Choi CH (2020) Computation of molecular electron affinities using an ensemble density functional theory method. J Phys Chem A 124(38):7795–7804. https://doi.org/10.1021/acs.jpca.0c06976
Lu T, Manzetti S (2014) Wavefunction and reactivity study of benzo[a]pyrene diol epoxide and its enantiomeric forms. Struct Chem 25(5):1521–1533. https://doi.org/10.1007/s11224-014-0430-6
Antal Z, Warburton PL, Mezey PG (2014) Electron density shape analysis of a family of through-space and through-bond interactions. Phys Chem Chem Phys 16(3):918–932. https://doi.org/10.1039/c3cp53954g
Darabdhara G, Das MR (2019) Dual responsive magnetic Au@Ni nanostructures loaded reduced graphene oxide sheets for colorimetric detection and photocatalytic degradation of toxic phenolic compounds. J Hazard Mater 368:365–377. https://doi.org/10.1016/j.jhazmat.2019.01.010
Lin Z, Xiao Y, Yin Y, Hu W, Liu W, Yang H (2014) Facile synthesis of enzyme-inorganic hybrid nanoflowers and its application as a colorimetric platform for visual detection of hydrogen peroxide and phenol. ACS Appl Mater Interfaces 6(13):10775–10782. https://doi.org/10.1021/am502757e
Liu J, Zhang W, Peng M, Ren G, Guan L, Li K, Lin Y (2020) Zif-67 as a template generating and tuning “raisin pudding”-type nanozymes with multiple enzyme-like activities: toward online electrochemical detection of 3,4-dihydroxyphenylacetic acid in living brains. ACS Appl Mater Interfaces 12(26):29631–29640. https://doi.org/10.1021/acsami.0c05667
Kaur M, Mehta SK, Kansal SK (2017) Nitrogen doped graphene quantum dots: efficient fluorescent chemosensor for the selective and sensitive detection of 2,4,6-trinitrophenol. Sensors Actuators B Chem 245:938–945. https://doi.org/10.1016/j.snb.2017.02.026
Funding
This research was supported by the National Natural Science Foundation of China (NSFC, No. 21874109).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 3.04 mb)
Rights and permissions
About this article
Cite this article
Hu, C.Y., Jiang, Z.W., Huang, C.Z. et al. Cu2+-modified MOF as laccase-mimicking material for colorimetric determination and discrimination of phenolic compounds with 4-aminoantipyrine. Microchim Acta 188, 272 (2021). https://doi.org/10.1007/s00604-021-04944-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-021-04944-5