Abstract
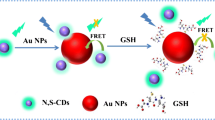
A convenient fluorometric method was developed for specific determination of DNA based on peptide nuclei acid (PNA)–regulated fluorescence resonance energy transfer (FRET) between carbon dots (CDs) and gold nanoparticles (AuNPs). In this system, CDs that display lake blue fluorescence with excitation/emission maxima at 345/445 nm were used as fluorometric reporter, while AuNPs were used as fluorescence nanoquencher. A neutral PNA probe, which is designed to recognize the target DNA, was used as a coagulant to control the dispersion and aggregation of AuNPs. Without DNA, PNA can induce immediate AuNP aggregation, thus leading to the recovery of the FRET-quenched fluorescence emission of CDs. However, the addition of the complementary target DNA can protect AuNPs from being aggregated due to the formation of DNA/PNA complexes, which subsequently produces a high fluorescence quenching efficiency of CDs by dispersed AuNPs. Under optimized conditions, quantitative evaluation of DNA was achieved in a linear range of 5–100 nM with a detection limit of 0.21 nM. This method exhibited an excellent specificity towards fully matched DNA. In addition, the application of this assay for sensitive determination of DNA in cell lysate demonstrates its potential for bioanalysis and biodetection.

A simple fluorometric biosensor for specific detection of DNA was developed based on peptide nuclei acid (PNA)–regulated fluorescence resonance energy transfer (FRET) between carbon dots (CDs) and gold nanoparticles (AuNPs).







Similar content being viewed by others
References
Bidard F-C, Weigelt B, Reis-Filho JS (2013) Going with the flow: from circulating tumor cells to DNA. Sci Transl Med 5:207–214
Fiala C, Diamandis EP (2018) Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med 16:166
Yuan L, Lin W, Zheng K, He L, Huang W (2013) Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev 42:622–661
Yang Y, Zhao Q, Feng W, Li F (2013) Luminescent chemodosimeters for bioimaging. Chem Rev 113:192–270
Wang L, Dong L, Liu G, Shen X, Wang J, Zhu C, Ding M, Wen Y (2019) Fluorometric determination of HIV DNA using molybdenum disulfide nanosheets and exonuclease III-assisted amplification. Mikrochim Acta 186:286
Mandal TK, Parvin N, Mishra K, Mohandoss S, Lee YR (2019) Sensitive and selective fluorometric determination of DNA by using layered hexagonal nanosheets of a covalent organic framework prepared from p-phenylenediamine and benzene-1,3,5-tricarboxaldehyde. Microchim Acta 186:833
Iwe I, Li Z, Huang J (2019) Graphene oxide and enzyme-assisted dual-cycling amplification method for sensitive fluorometric determination of DNA. Microchim Acta 186:716
Tyagi S, Kramer FR (1996) Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol 14:303–308
Yang CYJ, Medley CD, Tan WH (2005) Monitoring nucleic acids using molecular beacons. Curr Pharm Biotechnol 6:445–452
Piatek AS, Tyagi S, Pol AC, Telenti A, Miller LP, Kramer FR, Alland D (1998) Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat Biotechnol 16:359–363
Peng XH, Cao ZH, Xia JT, Carlson GW, Lewis MM, Wood WC, Yang L (2005) Real-time detection of gene expression in cancer cells using molecular beacon imaging: new strategies for cancer research. Cancer Res 65:1909–1917
Radi AE, Sanchez JLA, Baldrich E, O'Sullivan CK (2006) Reagentless, reusable, ultrasensitive electrochemical molecular beacon aptasensor. J Am Chem Soc 128:117–124
Boens N, Leen V, Dehaen W (2012) Fluorescent indicators based on BODIPY. Chem Soc Rev 41:1130–1172
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381
Sun Y-P, Zhou B, Lin Y, Wang W, Fernando KAS, Pathak P, Meziani MJ, Harruff BA, Wang X, Wang H, Luo PG, Yang H, Kose ME, Chen B, Veca LM, Xie S-Y (2006) Quantum-sized carbon dots for bright and colorful photoluminescence. J Am Chem Soc 128:7756–7757
Baker SN, Baker GA (2010) Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Ed 49:6726–6744
Xu Q, Gong Y, Zhang Z, Miao Y, Li D, Yan G (2019) Preparation of graphene oxide quantum dots from waste toner, and their application to a fluorometric DNA hybridization assay. Microchim Acta 186:483
Tang J, Kong B, Wu H, Xu M, Wang Y, Wang Y, Zhao D, Zheng G (2013) Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv Mater 25:6569–6574
Loo AH, Sofer Z, Bousa D, Ulbrich P, Bonanni A, Pumera M (2016) Carboxylic carbon quantum dots as a fluorescent sensing platform for DNA detection. ACS Appl Mater Interfaces 8:1951–1957
Li F, Pei H, Wang L, Lu J, Gao J, Jiang B, Zhao X, Fan C (2013) Nanomaterial-based fluorescent DNA analysis: a comparative study of the quenching effects of graphene oxide, carbon nanotubes, and gold nanoparticles. Adv Funct Mater 23:4140–4148
Shi J, Tian F, Lyu J, Yang M (2015) Nanoparticle based fluorescence resonance energy transfer (FRET) for biosensing applications. J Mater Chem B 3:6989–7005
Lee MH, Leu CC, Lin CC, Tseng YF, Lin HY, Yang CN (2019) Gold-decorated magnetic nanoparticles modified with hairpin-shaped DNA for fluorometric discrimination of single-base mismatch DNA. Microchim Acta 186:80
Bu D, Zhuang H, Yang G, Ping X (2014) An immunosensor designed for polybrominated biphenyl detection based on fluorescence resonance energy transfer (FRET) between carbon dots and gold nanoparticles. Sensors Actuators B Chem 195:540–548
Shi Y, Pan Y, Zhang H, Zhang Z, Li M-J, Yi C, Yang M (2014) A dual-mode nanosensor based on carbon quantum dots and gold nanoparticles for discriminative detection of glutathione in human plasma. Biosens Bioelectron 56:39–45
Deng J, Lu Q, Hou Y, Liu M, Li H, Zhang Y, Yao S (2015) Nanosensor composed of nitrogen-doped carbon dots and gold nanoparticles for highly selective detection of cysteine with multiple signals. Anal Chem 87:2195–2203
Wang B, Chen Y, Wu Y, Weng B, Liu Y, Lu Z, Li CM, Yu C (2016) Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens Bioelectron 78:23–30
Qu F, Huang W, You J (2018) A fluorescent sensor for detecting dopamine and tyrosinase activity by dual-emission carbon dots and gold nanoparticles. Colloids Surf B: Biointerfaces 162:212–219
Li J, Rao X, Xiang F, Wei J, Yuan M, Liu Z (2018) A photoluminescence "switch-on" nanosensor composed of nitrogen and sulphur co-doped carbon dots and gold nanoparticles for discriminative detection of glutathione. Analyst 143:2083–2089
Yang Y, Huo D, Wu H, Wang X, Yang J, Bian M, Ma Y, Hou C (2018) N, P-doped carbon quantum dots as a fluorescent sensing platform for carbendazim detection based on fluorescence resonance energy transfer. Sensors Actuators B Chem 274:296–303
Yang Y, Hou J, Huo D, Wang X, Li J, Xu G, Bian M, He Q, Hou C, Yang M (2019) Green emitting carbon dots for sensitive fluorometric determination of cartap based on its aggregation effect on gold nanoparticles. Microchim Acta 186:259
Qin X, Lu Y, Bian M, Xiao Z, Zhang Y, Yuan Y (2019) Influence of gold nanoparticles in different aggregation states on the fluorescence of carbon dots and its application. Anal Chim Acta 1091:119–126
Xu S, Zhang F, Xu L, Liu X, Ma P, Sun Y, Wang X, Song D (2018) A fluorescence resonance energy transfer biosensor based on carbon dots and gold nanoparticles for the detection of trypsin. Sensors Actuators B Chem 273:1015–1021
Wang W, Wang Y, Pan H, Cheddah S, Yan C (2019) Aptamer-based fluorometric determination for mucin 1 using gold nanoparticles and carbon dots. Microchim Acta 186:544
Zhong D, Yang K, Wang Y, Yang X (2017) Dual-channel sensing strategy based on gold nanoparticles cooperating with carbon dots and hairpin structure for assaying RNA and DNA. Talanta 175:217–223
Qaddare SH, Salimi A (2017) Amplified fluorescent sensing of DNA using luminescent carbon dots and AuNPs/GO as a sensing platform: a novel coupling of FRET and DNA hybridization for homogeneous HIV-1 gene detection at femtomolar level. Biosens Bioelectron 89:773–780
Krawczak M (1990) The mutational spectrum of single base-pair substitutions causing human genetic disease: patterns and predictions. Hum Genet 85:55–74
Krawczak M, Cooper DN (1996) Single base-pair substitutions in pathology and evolution: two sides to the same coin. Hum Mutat 8:23–31
Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NST, Abeysinghe S, Krawczak M, Cooper DN (2003) Human gene mutation database (HGMD (R)): 2003 update. Hum Mutat 21:577–581
Egholm M, Buchardt O, Nielsen PE, Berg RH (1992) Peptide nucleic-acids (PNA)-oligonucleotide analogs with an achiral peptide backbone. J Am Chem Soc 114:1895–1897
Su X, Kanjanawarut R (2009) Control of metal nanoparticles aggregation and dispersion by PNA and PNA-DNA complexes, and its application for colorimetric DNA detection. ACS Nano 3:2751–2759
Xing S, Xu X, Fu P, Xu M, Gao T, Zhang X, Zhao C (2019) Colorimetric detection of single base-pair mismatches based on the interactions of PNA and PNA/DNA complexes with unmodified gold nanoparticles. Colloids Surf B: Biointerfaces 181:333–340
Xu W, Xing S, Xu X, Xu M, Fu P, Gao T, Zhao C (2018) Peptide nucleic acid-assisted label-free detection of single-nucleotide polymorphisms based on light scattering of carbon nanotubes. ACS Omega 3:17835–17841
Zhao C, Hoppe T, Setty MKHG, Murray D, Chun T-W, Hewlett I, Appella DH (2014) Quantification of plasma HIV RNA using chemically engineered peptide nucleic acids. Nat Commun 5:5079
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of au colloid monolayers. Anal Chem 67:735–743
Zeng P, Hou P, Jing CJ, Huang CZ (2018) Highly sensitive detection of hepatitis C virus DNA by using a one-donor-four-acceptors FRET probe. Talanta 185:118–122
Bao B, Pan Y, Gu B, Chen J, Xu Y, Su P, Liu Y, Tong L, Wang L (2018) Highly sensitive detection of nucleic acids using a cascade amplification strategy based on exonuclease III-assisted target recycling and conjugated polyelectrolytes. Analyst 143:4267–4272
Meng Y, Liu P, Zhou W, Ding J, Liu J (2018) Bioorthogonal DNA adsorption on polydopamine nanoparticles mediated by metal coordination for highly robust sensing in serum and living cells. ACS Nano 12:9070–9080
Li Y, Sun L, Qian J, Long L, Li H, Liu Q, Cai J, Wang K (2017) Fluorescent "on-off-on" switching sensor based on CdTe quantum dots coupled with multiwalled carbon nanotubes@graphene oxide nanoribbons for simultaneous monitoring of dual foreign DNAs in transgenic soybean. Biosens Bioelectron 92:26–32
Qian ZS, Shan XY, Chai LJ, Ma JJ, Chen JR, Feng H (2014) DNA nanosensor based on biocompatible graphene quantum dots and carbon nanotubes. Biosens Bioelectron 60:64–70
Funding
This work was supported by Ningbo Natural Science Foundation (2017C110020, 2018A610318, 2019C50039) and funds from Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 16686 kb)
Rights and permissions
About this article
Cite this article
Gao, T., Xing, S., Xu, M. et al. A peptide nucleic acid–regulated fluorescence resonance energy transfer DNA assay based on the use of carbon dots and gold nanoparticles. Microchim Acta 187, 375 (2020). https://doi.org/10.1007/s00604-020-04357-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-04357-w