Abstract
A new sorbent was synthesized for restricted-access matrix solid phase extraction (RAM-SPE) of the diabetes drugs metformin (MET) and glimepiride (Glim). Mesoporous silica layers were placed on Fe3O4-magnetized graphene modified with sulfo-functionalized pore walls (denoted as magG@mSiO2-SO3H composites). The composites have a large specific surface (173 m2·g−1), appropriate pore sizes (typically 3.7 nm), and sulfo-functionalized pore walls. Magnetic separation can be accomplished within 10 s. The unique properties of the composites allow both MET and Glim to be selectively and quickly extracted from plasma sample. Following magnetic separation and elution by 50% aqueous acetonitrile with 4% ammonium solution, the two drugs were quantified by LC-MS/MS analysis. The assay has high selectivity, good linearity (2.5–4000 ng•mL−1 for MET and 0.02–1600 ng•mL−1 for Glim), a low detection limit (as low as 60 pg•mL−1 for MET and 1 pg•mL−1 for Glim), excellent recovery (above 92.2%), and good precision (RSDs <12%). The method was successfully applied in a pharmacokinetic study in beagle dogs.

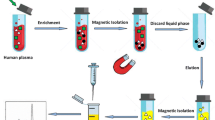
Schematic representation of the synthesis of sulfo-functionalized magnetic graphene/mesoporous silica composites, giving a material of type magG@mSiO2-SO3H. Results showed its great potential as a feasible and alternative adsorbent for the selective extraction of MET and Glim from complicated biological samples.




Similar content being viewed by others
References
Krentz AJ, Bailey CJ (2005) Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs 65(65):385–411
Khan AS, Mcloughney CR, Ahmed AB (2010) The effect of metformin on blood glucose control in overweight patients with type 1 diabetes. Diabet Med 23(10):1079–1084
Derosa G, Franzetti I, Gadaleta G, Ciccarelli L, Fogari R (2004) Metabolic variations with oral antidiabetic drugs in patients with type 2 diabetes: comparison between glimepiride and metformin. Diabetes Nutr Metab 17(3):143–150
Massibenedetti M (2003) Glimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experience. Clin Ther 25(3):799–816
Pareek A, Chandurkar NB, Salkar HR, Borkar MS, Tiwari D (2013) Evaluation of efficacy and tolerability of glimepiride and metformin combination: a multicentric study in patients with type-2 diabetes mellitus, uncontrolled on monotherapy with sulfonylurea or metformin. Am J Ther 20(1):41–47
Marques MAS, Soares ADS, Pinto OW, Barroso PTW, Pinto DP, Ferreira-Filho M, Werneck-Barroso E (2007) Simple and rapid method determination for metformin in human plasma using high performance liquid chromatography tandem mass spectrometry: application to pharmacokinetic studies. J Chromatogr B 852(1):308–316
Salem II, Idrees J, Al Tamimi JI (2004) Determination of glimepiride in human plasma by liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr B 799(1):103–109
Dotsikas Y, Kousoulos C, Tsatsou G, Loukas YL (2010) Development of a rapid method for the determination of glimepiride in human plasma using liquid-liquid extraction based on 96-well format micro-tubes and liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Sp 19(14):2055–2061
Hotha KK, Yarramu NR, Kandibedala T, Dasari VB, Vobalaboina V (2012) Simultaneous determination of atorvastatin and glimepiride by LC-MS/MS in human plasma and its application to a pharmacokinetic study. Am J Anal Chem 3(8):559–569
Ni XJ, Wang ZZ, Shang DW, Zhang M, Hu JQ, Qiu C, Wen YG (2014) Simultaneous determination of glimepiride and pioglitazone in human plasma by liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic study. J Chromatogr B 960(6):247–252
Zhang L, Yuan T, Zhang Z, Chen Y (2007) Simultaneous determination of metformin and rosiglitazone in human plasma by liquid chromatography/tandem mass spectrometry with electrospray ionization: application to a pharmacokinetic study. J Chromatogr B 854(1):91–98
Zhong G, Bi H, Zhou S, Chen X, Huang M (2010) Simultaneous determination of metformin and gliclazide in human plasma by liquid chromatography–tandem mass spectrometry: application to a bioequivalence study of two formulations in healthy volunteers. J Mass Spectrom 40(11):1462–1471
Elzanfaly ES, Abdel-Gawad SA, Elzanfaly ES, Abdel-Gawad SA, Elzanfaly ES, Abdel-Gawad SA (2017) Simultaneous quantification of ramipril, glimepiride and metformin in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry. J Appl Pharm Sci 7(7):062–069
Polagani SR, Pilli NR, Gajula R, Gandu V (2013) Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC-MS/MS and its application to a human pharmacokinetic study. J Pharm Anal 3(1):9–19
Sengupta P, Bhaumik U, Ghosh A, Sarkar AK, Chatterjee B, Bose A, Pal TK (2009) LC-MS-MS development and validation for simultaneous quantitation of metformin, glimepiride and pioglitazone in human plasma and its application to a bioequivalence study. Chromatographia 69(11–12):1243–1250
Bai J, Shi X, Du Y, Xiang B, Wang S, Cao D (2012) Liquid chromatography-electrospray ionization tandem mass spectrometry for simultaneous determination of metformin and glimepiride in beagle dog plasma and bioequivalence study. Chem Res Chinese U 28(3):399–405
Oertel R, Baldauf J, Rossmann J (2018) Development and validation of a hydrophilic interaction liquid chromatography-tandem mass spectrometry method for the quantification of the antidiabetic drug metformin and six others pharmaceuticals in wastewater. J Chromatogr A 1556:73–80
Musmade PB, Talole KB, Deshpande PB, Karthik A, Pathak SM, Pandey S, Udupa N (2011) Novel liquid chromatographic method for simultaneous estimation of pioglitazone and glimepiride in rat plasma by solid phase extraction: application to preclinical pharmacokinetic studies. Arzneimittelforschung 61(01):23–31
Feng J, He X, Liu X, Sun X, Li Y (2016) Preparation of magnetic graphene/mesoporous silica composites with phenyl-functionalized pore-walls as the restricted access matrix solid phase extraction adsorbent for the rapid extraction of parabens from water-based skin toners. J Chromatogr A 1465:20–29
Liu X, Feng J, Li Y (2018) Preparation of carbon-functionalized magnetic graphene/mesoporous silica composites for selective extraction of miglitol and voglibose in rat plasma. Talanta 182:405–413
Feng J, She X, He X, Zhu J, Li Y, Deng C (2018) Synthesis of magnetic graphene/mesoporous silica composites with boronic acid-functionalized pore-walls for selective and efficient residue analysis of aminoglycosides in milk. Food Chem 239:612–621
Liu X, Yu Y, Li Y, Zhang H, Ling J, Sun X, Feng J, Duan G (2014) Fluorocarbon-bonded magnetic mesoporous microspheres for the analysis of perfluorinated compounds in human serum by high-performance liquid chromatography coupled to tandem mass spectrometry. Anal Chim Acta 844:35–43
Niina M (2005) Development and validation for high selective quantitative determination of metformin in human plasma by cation exchanging with normal-phase LS/MS/MS. J Pharmaceu Biomed 36(5):1063–1072
Vesterqvist O, Nabbie F, Swanson B (1998) Determination of metformin in plasma by high-performance liquid chromatography after ultrafiltration. J Chromatogr B Biomed Sci Appl 716(1–2):299–304
European Medicines Agency. Guideline on bioanalytical method validation (EMEA/CHMP/EWP/192217/2009); 2011. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. Accessed Dec 2018
van der Graaff WNP, Olvera KG, Pidko EA, Hensen EJM (2014) Stability and catalytic properties of porous acidic (organo) silica materials for conversion of carbohydrates. J Mol Catal a Chem 388-389(s 388–389): 81-89
Bouchoucha M, Uzzan B, Cohen R (2011) Metformin and digestive disorders. Diabetes Metab 37(2):90–96
Acknowledgements
This work was supported by funds provided by the Natural Science Foundation of China (Project no. 21675034), the Natural Science Foundation of Shanghai (Project no. 16ZR1402300), the Ministry of Science and Technology of the People’s Republic of China (Grant no. 2018ZX09J18112), PDH-SPFDU Joint Research Fund (no. RHJJ2017-02), Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (No. PWR12014-06), Integrative Medicine special fund of Shanghai Municipal Health Planning Committee (No. ZHYY-ZXYJHZX-2-201712) and Key studies (special) Department Fund of the Pudong New Area Health Planning Commission (No. PWZzk2017-03).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1.35 mb)
Rights and permissions
About this article
Cite this article
Jiang, J., She, X., Zhu, J. et al. A composite consisting of sulfo-functionalized magnetic graphene and mesoporous silica for extraction of metformin and glimepiride prior to their determination by liquid chromatography tandem mass spectrometry. Microchim Acta 186, 590 (2019). https://doi.org/10.1007/s00604-019-3693-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3693-1