Abstract
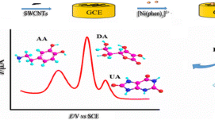
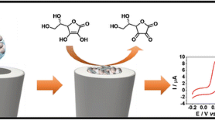
Electrochemical sensing of L-ascorbic acid (AA) is reported based on the use of a redox-active molybdophosphate film on a glassy carbon electrode (GCE). Molybdophosphate is formed by reacting hydroxyapatite nanoparticles with sodium molybdate. The modified GCE can be utilized for detection of AA, typically at a working potential of 0.4 V (vs. Ag/AgCl). The GCE has a decreased overpotential and enhanced sensitivity (219 μA·mM−1·cm−2). Response is linear in the 1 μM to 1.5 mM AA concentration range, and the limit of detection is 4 nM. The selectivity of this sensor makes it a useful tool for accurate determination of AA in practical samples as shown for a vitamin C tablet and for spiked beverages.

An electrochemical sensing platform is reported that is based on the use of a redox-active molybdophosphate film that was formed via reacting hydroxyapatite nanoparticles (HAP-NPs) with sodium molybdate. Graphical abstract contains poor quality of text inside the artwork. Please do not re-use the file that we have rejected or attempt to increase its resolution and re-save. It is originally poor, therefore, increasing the resolution will not solve the quality problem. We suggest that you provide us the original format. We prefer replacement figures containing vector/editable objects rather than embedded images. Preferred file formats are eps, ai, tiff and pdf.We have uploaded the graphical abstract as PDF format







Similar content being viewed by others
References
Gallarate M, Carlotti ME, Trotta M, Bovo S (1999) On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int J Pharm 188(2):233–241
Pisoschi AM, Pop A, Serban AI, Fafaneata C (2014) Electrochemical methods for ascorbic acid determination. Electrochim Acta 121:443–460
Arrigoni O, De Tullio MC (2002) Ascorbic acid: much more than just an antioxidant. Biochimica Et Biophysica Acta-General Subj 1569(1–3):1–9
Kalimuthu P, John SA (2009) Electropolymerized film of functionalized thiadiazole on glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Bioelectrochemistry 77(1):13–18
Massey LK, Liebman M, Kynast-Gales SA (2005) Ascorbate increases human oxaluria and kidney stone risk. J Nutr 135(7):1673–1677
Padayatty SJ, Katz A, Wang YH, Eck P, Kwon O, Lee JH, Chen SL, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22(1):18–35
Zuo X, Zhang H, Li N (2012) An electrochemical biosensor for determination of ascorbic acid by cobalt (II) phthalocyanine-multi-walled carbon nanotubes modified glassy carbon electrode. Sensor Actuat B-Chem 161(1):1074–1079
Suntornsuk L, Gritsanapun W, Nilkamhank S, Paochom A (2002) Quantitation of vitamin C content in herbal juice using direct titration. J Pharm Biomed Anal 28(5):849–855
Dai H, Wu XP, Wang YM, Zhou WC, Chen GN (2008) An electrochemiluminescent biosensor for vitamin C based on inhibition of luminol electrochemiluminescence on graphite/poly(methyl methacrylate) composite electrode. Electrochim Acta 53(16):5113–5117
Tai A, Gohda E (2007) Determination of ascorbic acid and its related compounds in foods and beverages by hydrophilic interaction liquid chromatography. J Chromatogr B-Anal Technol Biomed Life Sci 853(1–2):214–220
Wu X, Diao YX, Sun CX, Yang JH, Wang YB, Sun SN (2003) Fluorimetric determination of ascorbic acid with o-phenylenediamine. Talanta 59(1):95–99
Kumar SA, Lo PH, Chen SM (2008) Electrochemical selective determination of ascorbic acid at redox active polymer modified electrode derived from direct blue 71. Biosens Bioelectron 24(4):518–523
Wen D, Guo SJ, Dong SJ, Wang EK (2010) Ultrathin Pd nanowire as a highly active electrode material for sensitive and selective detection of ascorbic acid. Biosens Bioelectron 26(3):1056–1061
Khan A, Khan MI, Iqbal Z, Shah Y, Ahmad L, Nazir S, Watson DG, Khan JA, Nasir F, Khan A, Ismail (2011) A new HPLC method for the simultaneous determination of ascorbic acid and aminothiols in human plasma and erythrocytes using electrochemical detection. Talanta 84(3):789–801
Weng X, Cao Q, Liang L, Chen J, You C, Ruan Y, Lin H, Wu L (2013) Simultaneous determination of dopamine and uric acid using layer-by-layer graphene and chitosan assembled multilayer films. Talanta 117:359–365
Sha YF, Qian L, Ma Y, Bai HX, Yang XR (2006) Multilayer films of carbon nanotubes and redox polymer on screen-printed carbon electrodes for electrocatalysis of ascorbic acid. Talanta 70(3):556–560
Thangamuthu R, Senthil Kumar SM, Chandrasekara Pillai K (2007) Direct amperometric determination of l-ascorbic acid (vitamin C) at octacyanomolybdate-doped-poly(4-vinylpyridine) modified electrode in fruit juice and pharmaceuticals. Sensors Actuators B Chem 120(2):745–753
Zhang X, Cao Y, Yu S, Yang F, Xi P (2013) An electrochemical biosensor for ascorbic acid based on carbon-supported PdNi nanoparticles. Biosens Bioelectron 44:183–190
Xi L, Ren D, Luo J, Zhu Y (2010) Electrochemical analysis of ascorbic acid using copper nanoparticles/polyaniline modified glassy carbon electrode. J Electroanal Chem 650(1):127–134
Li Y, Li X, Wang D, Shen C, Yang M (2018) Hydroxyapatite nanoparticle based fluorometric turn-on determination of dipicolinic acid, a biomarker of bacterial spores. Microchim Acta 185(9):435
Li Y, Shen C, Li X, Yang M, Shao C (2018) Hydroxyapatite nanoparticle based fluorometric determination and imaging of cysteine and homocysteine in living cells. Microchim Acta 185(5):271
Zhang K, Zeng K, Shen C, Tian S, Yang M (2018) Determination of protein kinase A activity and inhibition by using hydroxyapatite nanoparticles as a fluorescent probe. Microchim Acta 185(4):225
Xiang W, Wang G, Cao S, Wang Q, Xiao X, Li T, Yang M (2018) Coupling antibody based recognition with DNA based signal amplification using an electrochemical probe modified with MnO2 nanosheets and gold nanoclusters: application to the sensitive voltammetric determination of the cancer biomarker alpha fetoprotein. Microchim Acta 185(7):335
Cao S, Wang Q, Xiao X, Li T, Yang M (2019) Electrochemical immunoassay for the tumor marker CD25 by coupling magnetic sphere-based enrichment and DNA based signal amplification. Microchim Acta 186: 352
Shen C, Liu S, Li X, Zhao D, Yang M (2018) Immunoelectrochemical detection of thehuman epidermal growth factor receptor 2 (HER2) via gold nanoparticle-based rolling circle amplification. Microchim Acta 185(12):547
Singh RK, Kim T-H, Patel KD, Kim J-J, Kim H-W (2014) Development of biocompatible apatite nanorod-based drug-delivery system with in situ fluorescence imaging capacity. J Mater Chem B 2(14):2039
Chai Y, Li X, Yang M (2019) Aptamer based determination of the cancer biomarker HER2 by using phosphate-functionalized MnO2nanosheets as the electrochemical probe. Microchim Acta 186:316
Wang G, Wang H, Cao S, Xiang W, Li T, Yang M (2019) Electrochemical determination of the activity and inhibition of telomerase based on the interaction of DNA with molybdate. Microchim Acta 186(2):96
Zhang H, Huang F, Xu S, Xia Y, Huang W, Li Z (2013) Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem Commun 30:46–50
Weng C-J, Hsu P-H, Hsu S-C, Chang C-H, Hung W-I, Wu P-S, Yeh J-M (2013) Synthesis of electroactive mesoporous gold–organosilica nanocomposite materials via a sol–gel process with non-surfactant templates and the electroanalysis of ascorbic acid. J Mater Chem B 1(38):4983
Fernandes DM, Costa M, Pereira C, Bachiller-Baeza B, Rodríguez-Ramos I, Guerrero-Ruiz A, Freire C (2014) Novel electrochemical sensor based on N-doped carbon nanotubes and Fe3O4 nanoparticles: simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. J Colloid Interface Sci 432:207–213
Gopalakrishnan A, Sha R, Vishnu N, Kumar R, Badhulika S (2018) Disposable, efficient and highly selective electrochemical sensor based on cadmium oxide nanoparticles decorated screen-printed carbon electrode for ascorbic acid determination in fruit juices. Nano-Struct Nano-Objects 16:96–103
Du J, Yue R, Ren F, Yao Z, Jiang F, Yang P, Du Y (2014) Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens Bioelectron 53:220–224
Jin L, Zhang Z, Zhuang Z, Meng Z, Li C, Shen Y (2016) PdPt bimetallic alloy nanowires-based electrochemical sensor for sensitive detection of ascorbic acid. RSC Adv 6(48):42008–42013
Abdelwahab AA, Kim D-M, Halappa NM, Shim Y-B (2013) A selective catalytic oxidation of ascorbic acid at the Aminopyrimidyl functionalized-conductive polymer electrode. Electroanalysis 25(5):1178–1184
Acknowledgments
The authors thank the support of this work by the National Natural Science Foundation of China (No. 21575165).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, S., Jiang, X. & Yang, M. Electrochemical sensing of L-ascorbic acid by using a glassy carbon electrode modified with a molybdophosphate film. Microchim Acta 186, 445 (2019). https://doi.org/10.1007/s00604-019-3562-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3562-y