Abstract
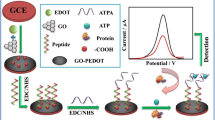
An antifouling electrochemical aptasensor for ATP is described that has a zwitterionic self-assembled sensing interface on a glassy carbon electrode modified with a reduced graphene oxide carbon nanofiber (GO-CNF). The GO-CNF was first modified by self-polymerization of dopamine which provided a platform for simultaneously self-assembly of the ATP aptamer and cysteine. By using hexacyanoferrate as the electrochemical probe, in the presence of ATP, the aptamer strands fold around ATP molecules, thus leading to the variation of the electrochemical signal. The aptasensor has a linear response in the 0.1 pM to 5 nM ATP concentration range, and a 13 fM lower detection limit. The electrode is strongly resistant to nonspecific adsorption and biofouling. This enabled the detection of ATP even in spiked human plasma.

An antifouling electrochemical aptasensor employing reduced graphene oxide carbon nanofiber as conductive substrate and zwitterionic cysteine as antifouling material for adenosine triphosphate detection.







Similar content being viewed by others
References
Deng J, Wang K, Wang M, Yu P, Mao L (2017) Mitochondria targeted nanoscale Zeolitic imidazole Framework-90 for ATP imaging in live cells. J Am Chem Soc 139:5877–5882
Qu F, Sun C, Lv X, You J (2018) A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim Acta 185:359
Liu X, Lin B, Yu Y, Cao Y, Guo M (2018) A multifunctional probe based on the use of labeled aptamer and magnetic nanoparticles for fluorometric determination of adenosine 5 '-triphosphate. Microchim Acta 185
Cheng X, Cen Y, Xu G, Wei F, Shi M, Xu X, Sohail M, Hu Q (2018) Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim Acta 185:144
El Kurdi R, Patra D (2018) Nanosensing of ATP by fluorescence recovery after surface energy transfer between rhodamine B and curcubit 7 uril-capped gold nanoparticles. Microchim Acta 185:349
Alberti D, van't Erve M, Stefania R, Ruggiero MR, Tapparo M, Geninatti Crich S, Aime S (2014) A quantitative Relaxometric version of the ELISA test for the measurement of cell surface biomarkers. Angew Chem Int Ed 53:3488–3491
Sun F, Ella-Menye J-R, Galvan DD, Bai T, Hung H-C, Chou Y-N, Zhang P, Jiang S, Yu Q (2015) Stealth surface modification of surface-enhanced Raman scattering substrates for sensitive and accurate detection in protein solutions. ACS Nano 9:2668–2676
He L, Pagneux Q, Larroulet I, Serrano AY, Pesquera A, Zurutuza A, Mandler D, Boukherroub R, Szunerits S (2017) Label-free femtomolar cancer biomarker detection in human serum using graphene-coated surface plasmon resonance chips. Biosens Bioelectron 89:606–611
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Bozokalfa G, Akbulut H, Demir B, Guler E, Gumus ZP, Odaci Demirkol D, Aldemir E, Yamada S, Endo T, Coskunol H, Timur S, Yagci Y (2016) Polypeptide functional surface for the aptamer immobilization: electrochemical cocaine biosensing. Anal Chem 88:4161–4167
Park KS (2018) Nucleic acid aptamer-based methods for diagnosis of infections. Biosens Bioelectron 102:179–188
Rapini R, Marrazza G (2017) Electrochemical aptasensors for contaminants detection in food and environment: recent advances. Bioelectrochemistry 118:47–61
Farzin L, Shamsipur M, Samandari L, Sheibani S (2018) Advances in the design of nanomaterial-based electrochemical affinity and enzymatic biosensors for metabolic biomarkers: a review. Microchim Acta 185:276
Zhang X, Song C, Yang K, Hong W, Lu Y, Yu P, Mao L (2018) Photoinduced regeneration of an aptamer-based electrochemical sensor for sensitively detecting adenosine triphosphate. Anal Chem 90:4968–4971
Nowinski AK, Sun F, White AD, Keefe AJ, Jiang S (2012) Sequence, structure, and function of peptide self-assembled monolayers. J Am Chem Soc 134:6000–6005
Schlenoff JB (2014) Zwitteration: coating surfaces with Zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30:9625–9636
Shih Y-J, Chang Y, Quemener D, Yang H-S, Jhong J-F, Ho F-M, Higuchi A, Chang Y (2014) Hemocompatibility of Polyampholyte copolymers with well-defined charge Bias in human blood. Langmuir 30:6489–6496
Wang P, Yang J, Zhou B, Hu Y, Xing L, Xu F, Shen M, Zhang G, Shi X (2017) Antifouling manganese oxide nanoparticles: synthesis, characterization, and applications for enhanced MR imaging of tumors. ACS Appl Mater Interfaces 9:47–53
Lin P, Chuang T-L, Chen PZ, Lin C-W, Gu FX (2019) Low-fouling characteristics of ultrathin Zwitterionic cysteine SAMs. Langmuir 35:1756–1767
Shevate R, Kumar M, Karunakaran M, Hedhili MN, Peinemann K-V (2017) Polydopamine/cysteine surface modified isoporous membranes with self-cleaning properties. J Membr Sci 529:185–194
Li P, Cai X, Wang D, Chen S, Yuan J, Li L, Shen J (2013) Hemocompatibility and anti-biofouling property improvement of poly(ethylene terephthalate) via self-polymerization of dopamine and covalent graft of zwitterionic cysteine. Colloids Surf B: Biointerfaces 110:327–332
Zhu C, Yang G, Li H, Du D, Lin Y (2015) Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal Chem 87:230–249
Liu Y, Ai K, Lu L (2014) Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chem Rev 114:5057–5115
Mo R, Jiang T, DiSanto R, Tai W, Gu Z (2014) ATP-triggered anticancer drug delivery. Nat Commun 5:3364
Biniuri Y, Albada B, Willner I (2018) Probing ATP/ATP-aptamer or ATP-aptamer mutant complexes by microscale thermophoresis and molecular dynamics simulations: discovery of an ATP-aptamer sequence of superior binding properties. J Phys Chem B 122:9102–9109
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3:101–105
Wang G, Xu Q, Liu L, Su X, Lin J, Xu G, Luo X (2017) Mixed self-assembly of polyethylene glycol and aptamer on Polydopamine surface for highly sensitive and low-fouling detection of adenosine triphosphate in complex media. ACS Appl Mater Interfaces 9:31153–31160
Wen Y-F, Cao X, Yang Y-G, Li H, Guo J-Q, Liu L (2008) Carbonization of pre-oxidized polyacrylonitrile fibers. Carbon 46:2
Wang G, Han R, Su X, Li Y, Xu G, Luo X (2017) Zwitterionic peptide anchored to conducting polymer PEDOT for the development of antifouling and ultrasensitive electrochemical DNA sensor. Biosens Bioelectron 92:396–401
Labib M, Sargent EH, Kelley SO (2016) Electrochemical methods for the analysis of clinically relevant biomolecules. Chem Rev 116:9001–9090
Llaudet E, Hatz S, Droniou M, Dale N (2005) Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem 77:3267–3273
Lu L, Si JC, Gao ZF, Zhang Y, Lei JL, Luo HQ, Li NB (2015) Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNAzyme-amplified electrochemical detection. Biosens Bioelectron 63:14–20
Lu L-M, Zhang X-B, Kong R-M, Yang B, Tan W (2011) A ligation-triggered DNAzyme Cascade for amplified fluorescence detection of biological small molecules with zero-background signal. J Am Chem Soc 133:11686–11691
Iliuk AB, Hu L, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Wang G, Su X, Xu Q, Xu G, Lin J, Luo X (2018) Antifouling aptasensor for the detection of adenosine triphosphate in biological media based on mixed self-assembled aptamer and zwitterionic peptide. Biosens Bioelectron 101:129–134
Acknowledgements
This work acknowledges support from the National Natural Science Foundation of China (No. 21375045) and Natural Science Foundation of Jilin Province (No. 20180101195JC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 2.44 mb)
Rights and permissions
About this article
Cite this article
Zhang, T., Xu, H., Xu, Z. et al. A bioinspired antifouling zwitterionic interface based on reduced graphene oxide carbon nanofibers: electrochemical aptasensing of adenosine triphosphate. Microchim Acta 186, 240 (2019). https://doi.org/10.1007/s00604-019-3343-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3343-7