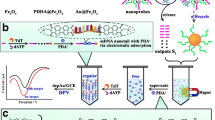
Abstract
An electrochemical method is described for the sensitive detection of the activity of the enzyme T4 polynucleotide kinase (PNK) by using a DNA functionalized porphyrinic metal-organic framework (L/(Fe-P)n-MOF). In the presence of PNK, the hairpin oligonucleotide (HP1) becomes phosphorylated, and the trigger is released by lambda exonuclease (λ exo). The trigger DNA hybridizes with hairpin probe (immobilized on the gold electrode) to form a nicking endonuclease cleavage site. Thus, a single-strand capture probe is employed to hybridize with L/(Fe-P)n-MOF. The (Fe-P)n-MOF is a peroxidase mimicking material with high catalytic efficiency. By using this amplification strategy, an electrochemical signal is procured that allows for the determination of T4 PNK in the 1.0 mU·mL−1 to 1.0 U·mL−1 with a detection limit of 0.62 mU·mL−1. The method is selective and can be used to screen for enzyme inhibitors. Conceivably, the (Fe-P)n-MOF can also be used to detect other analytes via its peroxidase-mimicking activity.

Schematic presentation of T4 polynucleotide kinase (PNK) detection. Two hairpin DNAs (HP) and a porphyrinic metal-organic framework with peroxidase-mimicking activity are used. The detection limit is 0.62 mU mL−1 with enzyme assisted signal amplification. This method is selective and can be used to screen for enzyme inhibitors.




Similar content being viewed by others
References
Ma CB, Yeung ES (2010) Highly sensitive detection of DNA phosphorylation by counting single nanoparticles. Anal Bioanal Chem 397:2279–2284
Tang ZW, Wang KM, Tan WH, Ma CB, Li J, Liu LF, Guo QP, Meng XX (2005) Real-time investigation of nucleic acids phosphorylation process using molecular beacons. Nucleic Acids Res 33:e97
Henner WD, Rodriguez LO, Hecht SM, Haseltine WA (1983) Gamma ray induced deoxyribonucleic acid strand breaks. 3′ Glycolate termini. J Biol Chem 258:711–713
Torriglia A, Perani P, Brossas JY, Chaudun E, Treton J, Courtois Y, Counis MF (1998) L-DNase II, a molecule that links proteases and endonucleases in apoptosis, derives from the ubiquitous serpin leukocyte elastase inhibitor. Mol Cell Biol 18:3612–3619
Lown JW, Mclaughlin LW (1979) Nitrosourea-induced DNA single-strand breaks. Biochem Pharmacol 28:1631–1638
Chen F, Zhao Y, Qi L, Fan C (2013) One-step highly sensitive florescence detection of T4 polynucleotide kinase activity and biological small molecules by ligation-nicking coupled reaction-mediated signal amplification. Biosens Bioelectron 47:218–224
Bernstein NK, Hammel M, Mani RS, Weinfeld M, Pelikan M, Tainer JA (2009) Mechanism of DNA substrate recognition by the mammalian DNA repair enzyme, polynucleotide kinase. Nucleic Acids Res 37:6161–6173
Odell M, Shuman S (1999) Footprinting of Chlorella virus DNA ligase bound at a nick in duplex DNA. J Biol Chem 274:14032–14039
Phillips DH, Arlt VM (2007) The 32P-postlabeling assay for DNA adducts. Nat Protoc 2:2772–2781
Jiang C, Yan C, Jiang J, Yu R (2013) Colorimetric assay for T4 polynucleotide kinase activity based on the horseradish peroxidase-mimicking DNAzyme combined with λ exonuclease cleavage. Anal Chim Acta 766:88–93
Lian S, Liu C, Zhang X, Wang H, Li Z (2015) Detection of T4 polynucleotide kinase activity based on cationic conjugated polymer-mediated fluorescence resonance energy transfer. Biosens Bioelectron 66:316–320
Zhu ZM, Yu RQ, Chu X (2014) Amplified fluorescence detection of T4 polynucleotide kinase activity and inhibition via a coupled λ exonuclease reaction and exonuclease III-aided trigger DNA recycling. Anal Methods 6:6009–6014
Guo Y, Wang Q, Wang Z, Chen X, Xu L, Hu J, Pei R (2015) Label-free detection of T4 DNA ligase and polynucleotide kinase activity based on toehold-mediated strand displacement and split G-quadruplex probes. Sensors Actuators B Chem 214:50–55
Song C, Yang X, Wang K, Wang Q, Liu J, Huang J, He L, Liu P, Qing Z, Liu W (2015) A sensitive detection of T4 polynucleotide kinase activity based on β-cyclodextrin polymer enhanced fluorescence combined with an exonuclease reaction. Chem Commun 51:1815–1818
Wang Y, Wu Y, Wang Y, Zhou B, Wu S (2015) A sensitive immobilization-free electrochemical assay for T4PNK activity based on exonuclease III-assisted recycling. RSC Adv 5:75348–75353
Zhang Q, Li Z, Zhou Y, Li X, Li B, Yin H, Ai S (2016) Electrochemical biosensors for polynucleotide kinase activity assay and inhibition screening based on phosphorylation reaction triggered λ exonuclease and exonuclease I cleavage. Sensors Actuators B 225:151–157
Hou T, Wang X, Liu X, Pan C, Li F (2014) Sensitive electrochemical assay for T4 polynucleotide kinase activity based on dual-signaling amplification coupled with exonuclease reaction. Sensors Actuators B Chem 202:588–593
Yu LQ, Yan XP (2013) Covalent bonding of zeolitic imidazolate framework-90 to functionalized silica fibers for solid-phase microextraction. Chem Commun 49:2142–2144
Zhang JW, Zhang HT, Du ZY, Wang X, Yu SH, Jiang HL (2014) Water-stable metal-organic frameworks with intrinsic peroxidase-like catalytic activity as a colorimetric biosensing platform. Chem Commun 50:1092–1094
Liu YL, Zhao XJ, Yang XX, Li YF (2013) A nanosized metal-organic framework of Fe-MIL-88NH2 as a novel peroxidase mimic used for colorimetric detection of glucose. Analyst 138:4526–4531
Chen Y, Hoang T, Ma SQ (2012) Biomimetic catalysis of a porous iron-based metal-metalloporphyrin framework. Inorg Chem 51:12600–12602
Nasir M, Nawaz MH, Yaqub M, Hayat A, Rahim A (2017) An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchim Acta 184:323–342
Cui L, Wu J, Li J, Ju H (2015) Electrochemical sensor for Lead cation sensitized with a DNA functionalized Porphyrinic metal-organic framework. Anal Chem 87:10635–10641
Liu G, Wan Y, Gau V, Zhang J, Wang L, Song S, Fan C (2008) An enzyme-based E-DNA sensor for sequence-specific detection of Femtomolar DNA targets. J Am Chem Soc 130:6820–6825
Chen JH, Zhang J, Guo Y, Li J, Fu FF, Yang HH, Chen GN (2011) An ultrasensitive electrochemical biosensor for detection of DNA species related to oral cancer based on nuclease-assisted target recycling and amplification of DNAzyme. Chem Commun 47:8004–8006
Fourmond V, Lautier T, Baffert C (2009) Correcting for Electrocatalyst desorption and inactivation in Chronoamperometry experiments. Anal Chem 81:2962–2968
Le’ger C, Bertrand P (2008) Direct electrochemistry of redox enzymes as a tool for mechanistic studies. Chem Rev 108:2379–2438
Vincent KA, Parkin A, Armstrong FA (2007) Investigating and exploiting the Electrocatalytic properties of hydrogenases. Chem Rev 107:4366–4413
Le’ger C, Dementin S, Bertrand P, Rousset M, Guigliarelli B (2004) Inhibition and aerobic inactivation kinetics of Desulfovibrio fructosovorans NiFe hydrogenase studied by protein film voltammetry. J Am Chem Soc 126:12162–12172
Almeida MG, Guigliarelli B, Bertrand P, Moura JJG, Moura IL’g C (2007) A needle in a haystack: the active site of the membrane-bound complex cytochrome c nitrite reductase. FEBS Lett 581:284–288
Liu S, Wang Y, Zhang C, Lin Y, Li F (2013) Homogeneous electrochemical aptamer-based ATP assay with signal amplification by exonuclease III assisted target recycling. Chem Commun 49:2335–2337
Wang Y, Wu Y, Wang Y, Zhou B, Wu S (2015) Analyzing clinical and electrophysiological characteristics of paroxysmal dyskinesia. RSC Adv 5:75348–75353
Zhang Q, Li Z, Zhou Y, Li X, Li B, Yin H, Ai S (2016) Electrochemical biosensors for polynucleotide kinase activity assay and inhibition screening based on phosphorylation reaction triggered λ exonuclease and exonuclease I cleavage. Sensors Actuators B 225:151–157
Feng C, Wang Z, Chen T, Chen X, Mao D, Zhao J, Li G (2018) A dual-enzyme-assisted three-dimensional DNA walking machine using T4 polynucleotide kinase as activators and application in polynucleotide kinase assays. Anal Chem 90:2810–2815
Lin L, Liu Y, Yan J, Wang X, Li J (2013) Sensitive nanochannel biosensor for T4 polynucleotide kinase activity and inhibition detection. Anal Chem 85:334–340
Wang LJ, Zhang Q, Tang B, Zhang CY (2017) Single-molecule detection of polynucleotide kinase based on phosphorylation-directed recovery of fluorescence quenched by au nanoparticles. Anal Chem 89:7255–7261
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21505082, 21705086, 21775080), a project of Shandong Province Higher Educational Science and Technology Program (Grant J16LC10), the Key Research and Development project of Shandong Province (No. 2017GSF221004, No. 2018GGX102001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 975 kb)
Rights and permissions
About this article
Cite this article
Song, W., Yin, W., Zhang, Z. et al. A DNA functionalized porphyrinic metal-organic framework as a peroxidase mimicking catalyst for amperometric determination of the activity of T4 polynucleotide kinase. Microchim Acta 186, 149 (2019). https://doi.org/10.1007/s00604-019-3269-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3269-0