Abstract
A method is described for multiple monolithic fiber solid-phase microextraction of five sterol and steroid hormones from complex food samples. A composite was prepared from graphene oxide, a metal-organic framework (ZIF-8) and a molecularly imprinted polymers was deposited on a single thin fiber. Four thin fibers were combined to obtain a fiber bundle. The nanocomposite was characterized by Fourier transform infrared spectroscopy, powder X-ray diffraction, scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy. The parameters affecting the extraction were optimized by Box-Behnken design. Following elution with methanol, the analytes progesterone, testosterone, β-sitosterol, cholesterol and campesterol were quantified via HPLC. Response is linear in the 0.01–1000 μg L−1 concentration range, and limits of detection range from 3 to 5 ng L−1. The method was successfully applied to the determination of the five analytes in spiked samples of white meat, egg yolks and vegetables. The relative mean recoveries ranged from 95.0% to 101.0%.

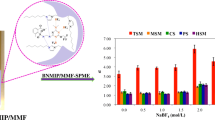
Schematic presentation of a monolith molecularly imprinting polymer (MIP) fiber fabrication for solid phase microextraction (SPME) of sterol and steroid hormones. The fiber was synthesis using graphene oxide and metal-organic framework, ZIF-8, composite by imprinting technique and progesterone as template. Four fibers were combined to obtain a fiber bundle. Then, multiple fiber solid-phase microextraction was employed for determination of analytes by coupling with HPLC/UV detection.





Similar content being viewed by others
References
Almeida C, Nogueira JMF (2006) Determination of steroid sex hormones in water and urine matrices by stir bar sorptive extraction and liquid chromatography with diode array detection. J Pharm Biomed Anal 41:1303–1311
Chang YC, Li Ch M, Li LA, Jong SB, Liao PC, Chang LW (2003) Quantitative measurement of male steroid hormones using automated on-line solid phase extraction-liquid chromatography-tandem mass spectrometry and comparison with radioimmunoassay. Analyst 128:363–368
Alonso RG, Espond SM, Ferrer ZS, Rodriguez JJ (2014) Liquid chromatography methodologies for the determination of steroid hormones in aquatic environmental systems. Trends Env Anal Chem 3–4:14–27
Arevalo FJ, Messina GA, Molina PG, Zon MA, Rabab J, Fernindez H (2010) Determination of progesterone (P4) from bovine serum samples using a microfluidic immunosensor system. Talanta 80:1986–1992
Kushnir MM, Rockwood AL, Roberts WL, Pattison EG, Bunker AM, Fitzgerald RL, Mikel AW (2006) Performance characteristics of a novel tandem mass spectrometry assay for serum testosterone. Clin Chem 52:120–128
Christiansen L, Karjalainen M, Seppanen-Laakso T, Hiltunen R, Yliruusi J (2003) Effect of β-sitosterol on precipitation of cholesterol from non-aqueous and aqueous solutions. Int J Pharm 254:155–166
Marangoni F, Poli A (2010) Phytosterols and cardiovascular health. Pharmacol Res 61:193–199
Rosenblat M, Volkova N, Aviram M (2013) Pomegranate phytosterol (β-sitosterol) and polyphenolic antioxidant (punicalagin) addition to statin, significantly protected against macrophage foam cells formation. Atherosclerosis 226:110–117
Puoci F, Curcio M, Cirillo G, Iemma F, Spizzirri UG, Picci N (2008) Molecularly imprinted solid-phase extraction for cholesterol determination in cheese products. Food Chem 106:836–842
Hum Y, Zhang Z (2008) Determination of free cholesterol based on a novel flow-injection chemiluminescence method by immobilizing enzyme. Luminescence 23:338–343
Hu Y, Wang Y, Chen X, Hu Y, Li G (2010) A novel molecularly imprinted solid-phase microextraction fiber coupled with high performance liquid chromatography for analysis of trace estrogens in fishery samples. Talanta 80:2099–2105
Lucci P, Nunez O, Galceran MT (2011) Solid-phase extraction using molecularly imprinted polymer for selective extraction of natural and synthetic estrogens from aqueous samples. J Chromatogr A 1218:4828–4833
Morales TV, Ferrer ZS, Rodriguez JJS (2012) Development and optimization of an on-line solid phase extraction coupled to ultra-high-performance liquid chromatography–tandem mass spectrometry methodology for the simultaneous determination of endocrine disrupting compounds in wastewater samples. J Chromatogr A 1230:66–76
Chi C, Chang S, Huang D (2010) Determination of the steroid hormone levels in water samples by dispersive liquid–liquid microextraction with solidification of a floating organic drop followed by high-performance liquid chromatography. Anal Chim Acta 662:39–43
Razmkhah K, Sereshti H, Soltani S, Nodeh HR (2018) Extraction and determination of three steroid molecules in milk using functionalized magnetic carbon nanotube-based solid phase extraction coupled with HPLC. Food Anal Methods 11:3179–3189
Tolgyesi A, Verebey Z, Sharma VK, Kovacsics L, Fekete J (2010) Simultaneous determination of corticosteroids, androgens, and progesterone in river water by liquid chromatography–tandem mass spectrometry. Chemosphere 78:972–979
Abdel-Khalik J, Bjorklund E, Hansen M (2013) Development of a solid phase extraction method for the simultaneous determination of steroid hormones in H295R cell line using liquid chromatography–tandem mass spectrometry. J Chromatogr B 935:61–69
Bowden JA, Colosi DM, Mora-Montero DC, Garrett TJ, Yost RA (2009) Enhancement of chemical derivatization of steroids by gas chromatography/mass spectrometry (GC/MS). J Chromatogr B 877:3237–3242
Basheer C, Balasubramanian R, Lee HK (2003) Determination of organic micropollutants in rain water using hollow fiber membrane/liquid-phase microextraction combined with gas chromatography–mass spectrometry. J Chromatogr A 1016:11–20
Zhang R, Li N, Ch W, Bai Y, Ren R, Sh G, Yu W, Zhao T, Zhang H (2011) Ionic liquid foam floatation coupled with solid phase extraction for separation and determination of hormones by high-performance liquid chromatography. Anal Chim Acta 704:98–109
Liu H, Chen L, Ding J (2017) A core-shell magnetic metal organic framework of type Fe3O4@ZIF-8 for the extraction of tetracycline antibiotics from water samples followed by ultra-HPLC-MS analysis. Microchim Acta 184:4091–4098
Peng J, Tian H, Du Q, Hui X, He H (2018) A regenerable sorbent composed of a zeolite imidazolate framework (ZIF-8), Fe3O4 and graphene oxide for enrichment of atorvastatin and simvastatin prior to their determination by HPLC. Microchim Acta 185:141–149
Li W, Zhang Y, Li Q, Zhang G (2015) Metal-organic framework composite membranes: synthesis and separation applications. Chem Eng Sci 135:232–257
Han T, Xiao Y, Tong M, Huang H, Liu D, Wang L, Zhong C (2015) Synthesis of CNT@MIL-68 (Al) composites with improved adsorption capacity for phenol in aqueous solution. Chem Eng J 275:134–141
Mirzajani R, Kardani F (2016) Fabrication of ciprofloxacin molecular mprinted polymer coating on a stainless steel wire as a selective solid-phase microextraction fiber for sensitive determination of fluoroquinolones in biological fluids and tablet formulation using HPLC-UV detection. J Pharm Biomed Anal 122:98–109
Su S, Chen B, He M, Hu B (2014) Graphene oxide–silica composite coating hollow fiber solid phase microextraction online coupled with inductively coupled plasma mass spectrometry for the determination of trace heavy metals in environmental water samples. Talanta 123:1–9
Zhao YG, Zhou LX, Pan SD, Zhan PP, Chen XH, Jin MC (2014) Fast determination of 22 sulfonamides from chicken breast muscle using core–shell nanoring amino-functionalized superparamagnetic molecularly imprinted polymer followed by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1345:17–28
Chen J, Yao B, Li C, Shi G (2013) An improved hummers method for eco-friendly synthesis of graphene oxide. Carbon 6 4:2 2 5–2 2 9
Xiang Z, Peng X, Cheng X, Li X, Cao D (2011) CNT@ Cu3(BTC)2 and metal- organic frameworks for separation of CO2/CH4 mixture. J Phys Chem 115:19864–19871
Ferreira SLC, Bruns RE, Ferreira HS, Matos GD, David JM, Brandao GC, da Silva EGP, Portugal LA, dos Reis PS, Souza AS, dos Santos WNL (2007) Box-Behnken design: an alternative for the optimization of analytical methods. Anal Chim Acta 597:179–186
Ribeiro JS, Teofilo RF, Augusto F, Ferreira MMC (2010) Simultaneous optimization of the microextraction of coffee volatiles using response surface methodology and principal component analysis. Chemom Intell Lab Syst 102:45–52
Kozlik P, Bosakova Z, Tesarova E, Coufal P, Cabala R (2011) Development of a solid-phase extraction with capillary liquid chromatography tandem mass spectrometry for analysis of estrogens in environmental water samples. J Chromatogr A 1218:2127–2132
Nieto A, Borrull F, Pocurull E, Marce RM (2008) Determination of natural and synthetic estrogens and their conjugates in sewage sludge by pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1213:224–230
Shishov A, Sviridov I, Timofeeva I, Chibisova N, Moskvin L, Bulatov A (2017) An effervescence tablet-assisted switchable solvent-basedmicroextraction: on-site preconcentration of steroid hormones in water samples followed by HPLC-UV determination. J Mol Liq 247:246–253
Gao G, Li S, Li S, Zhao L, Wang T, Hou X (2018) Development and application of vortex-assisted membrane extraction based on metal organic framework mixed-matrix membrane for the analysis of estrogens in human urine. Anal Chim Acta 22:1–9
Acknowledgements
The Authors greatly appreciate the financial support of this work by Shahid Chamran University of Ahvaz Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1.05 mb)
Rights and permissions
About this article
Cite this article
Mirzajani, R., Kardani, F. & Ramezani, Z. A nanocomposite consisting of graphene oxide, zeolite imidazolate framework 8, and a molecularly imprinted polymer for (multiple) fiber solid phase microextraction of sterol and steroid hormones prior to their quantitation by HPLC. Microchim Acta 186, 129 (2019). https://doi.org/10.1007/s00604-018-3217-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3217-4