Abstract
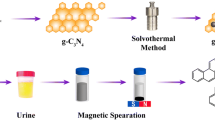
The authors have immobilized nanowires made from zirconium glycerolate (ZrGly) on magnetite (Fe3O4) nanoparticles by applying a solvothermal growth process using metal-glycerolate as a precursor. The structure and the dissolution-recrystallization mechanism of the resulting Fe3O4@ZrGly composite were investigated by attenuated total reflection-FTIR, energy-dispersive X-ray analysis, thermogravimetric analysis and solid-state cross polarization/magic angle spinning 13C NMR spectroscopy. The interaction between the zirconium glycerolate in Fe3O4@ZrGly and cis-diols leads to efficient adsorption of riboncleosides which then can be quantified by HPLC with UV detection. The sorbent was successfully applied to the selective enrichment of adenosine, cytidine, uridine and guanosine from spiked human urine samples. The detection limit of the method is in the range from 1.7 to 19 ng·mL−1 of nucleosides in spiked human urine, with relative standard deviations of lower than 12.4% and recoveries ranging from 90.6 to 113%.

Fe3O4@ZrGly with high selectivity towards ribonucleosides was designed and applied for quantitation of urinary ribonucleosides.




Similar content being viewed by others
References
Shao Y, Zhu B, Zheng R, Zhao X, Yin P, Lu X, Jiao B, Xu G, Yao Z (2015) Development of urinary pseudotargeted LC-MS-based metabolomics method and its application in hepatocellular carcinoma biomarker discovery. J Proteome Res 14(2):906–916
Kobayashi Y (2014) High-performance lectin affinity chromatography. Methods Mol Biol 1200:69–77
Boulton M, Jones A, Walker R (1971) Synthetic analogues of polynucleotides. VI. The synthesis of ribonucleoside dialdehyde derivatives of polyacrylic acid hydrazide and their interaction with polynucleotides. Biochim Biophys Acta 246(2):197–205. https://doi.org/10.1016/0005-2787(71)90128-6
Dai Q, Ma J, Ma S, Wang S, Li L, Zhu X, Qiao X (2016) Cationic ionic liquids organic ligands based metal-organic frameworks for fabrication of core-shell microspheres for hydrophilic interaction liquid chromatography. ACS Appl Mater Interfaces 8(33):21632–21639
Liu Y, Ren L, Liu Z (2011) A unique boronic acid functionalized monolithic capillary for specific capture, separation and immobilization of cis-diol biomolecules. Chem Commun (Camb) 47(17):5067–5069
Cheng T, Zhang Y, Liu X, Zhang X, Zhang H (2017) A filter paper coated with phenylboronic acid-modified mesoporous silica for enrichment of intracellular nucleosides prior to their quantitation by HPLC. Microchim Acta 184(10):4007–4013
Cheng T, Zhang Y, Liu X, Zhang X, Zhang H (2017) Surfactant assisted enrichment of nucleosides by using a sorbent consisting of magnetic polysulfone capsules and mesoporous silica nanoparticles modified with phenylboronic acid. Microchim Acta 184(1):271–278
Jiang G, Zhu W, Shen X, Xu L, Li X, Wang R, Liu C, Zhou X (2017) Colorimetric and visual determination of adenosine triphosphate using a boronic acid as the recognition element, and based on the deaggregation of gold nanoparticles. Microchim Acta 184(11):4305–4312
Li H, Zhu S, Cheng T, Wang S, Zhu B, Liu X, Zhang H (2016) Binary boronic acid-functionalized attapulgite with high adsorption capacity for selective capture of nucleosides at acidic pH values. Microchim Acta 183(5):1779–1786
Sun X, Ma R, Chen J, Shi Y (2017) Boronate-affinity based magnetic molecularly imprinted nanoparticles for the efficient extraction of the model glycoprotein horseradish peroxidase. Microchim Acta 184(10):3729–3737
Wang H, Feng W, Jia Q (2015) A graphene oxide functionalized with 3-aminophenylboronic acid for the selective enrichment of nucleosides, and their separation by capillary electrophoresis. Microchim Acta 182(1):185–192
Chu J, Qi C, Huang Y, Jiang H, Hao Y, Yuan B, Feng Y (2015) Metal oxide-based selective enrichment combined with stable isotope labeling-mass spectrometry analysis for profiling of ribose conjugates. Anal Chem 87(14):7364–7372
Ahmadi M, Elmongy H, Madrakian T, Abdel-Rehim M (2017) Nanomaterials as sorbents for sample preparation in bioanalysis: a review. Anal Chim Acta 958:1–21
Jamshaid T, Neto E, Eissa M, Zine N, Kunita M, El-Salhi A, Elaissari A (2016) Magnetic particles: from preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications. Trac-Trend Anal Chem 79:344–362
Park S, Choi K, Lee S, Oh I, Park S, Park H (2017) CNT branching of three-dimensional steam-activated graphene hybrid frameworks for excellent rate and cyclic capabilities to store lithium ions. Carbon 116:500–509
Qian X, Xu Q, Hang T, Shanmugam S, Li M (2017) Electrochemical deposition of Fe3O4 nanoparticles and flower-like hierarchical porous nanoflakes on 3D cu-cone arrays for rechargeable lithium battery anodes. Mater Design 121:321–334
Bian X, Hong K, Ge X, Song R, Liu L, Xu M (2015) Functional hierarchical nanocomposites based on ZnO nanowire and magnetic nanoparticle as highly active recyclable photocatalysts. J Phys Chem C 119(4):1700–1705
Yuan J, Schmalz H, Xu Y, Miyajima N, Drechsler M, Moeller M, Schacher F, Mueller A (2008) Room-temperature growth of uniform tellurium nanorods and the assembly of tellurium or Fe3O4 nanoparticles on the nanorods. Adv Mater 20(5):947–94+
Ding J, Gao Q, Luo D, Shi Z, Feng Y (2010) N-Octadecylphosphonic acid grafted mesoporous magnetic nanoparticle: preparation, characterization, and application in magnetic solid-phase extraction. J Chromatogr A 1217(47):7351–7358
Ren L, Liu Z, Dong M, Ye M, Zou H (2009) Synthesis and characterization of a new boronate affinity monolithic capillary for specific capture of cis-diol-containing compounds. J C hromatogr A 1216(23):4768
Tian G, Chen Y, Zhou W, Pan K, Tian C, Huang X, Fu H (2011) 3D hierarchical flower-like TiO2 nanostructure: morphology control and its photocatalytic property. CrystEngComm 13(8):2994–3000
Indran V, Syuhadazuhaimi N, Deraman M, Maniam G, Yusoff M, Yunhin TY, Ab. Rahim M (2014) An accelerated route of glycerol carbonate formation from glycerol using waste boiler ash as catalyst. RSC Adv 4 (48):25257
Remias R, Kukovecz A, Daranyi M, Kozma G, Varga S, Konya Z, Kiricsi I (2009) Synthesis of zinc glycerolate microstacks from a ZnO nanorod sacrificial template. Eur J Inorg Chem 24:3622–3627
Zhang J, Wang Y, He Y, Jiang T, Yang H, Tan X, Kang R, Yuan Y, Shi L (2010) Determination of urinary adenosine using resonance light scattering of gold nanoparticles modified structure-switching aptamer. Anal Biochem 397(2):212–217
Wang Y, Jiang X, Xia Y (2003) A solution-phase, precursor route to polycrystalline SnO2 nanowires that can be used for gas sensing under ambient conditions. J Am Chem Soc 125(52):16176–16177
Jiang X, Wang Y, Herricks T, Xia Y (2004) Ethylene glycol-mediated synthesis of metal oxide nanowires. J Mater Chem 14(4):695–703
Lopes T, Ribeiro M, Ming C, Grimaldi R, Goncalves L, Marsaioli A (2016) Comparison of the regiospecific distribution from triacylglycerols after chemical and enzymatic interesterification of high oleic sunflower oil and fully hydrogenated high oleic sunflower oil blend by carbon-13 nuclear magnetic resonance. Food Chem 212:641–647
Merchak N, Silvestre V, Loquet D, Rizk T, Akoka S, Bejjani J (2017) A strategy for simultaneous determination of fatty acid composition, fatty acid position, and position-specific isotope contents in triacylglycerol matrices by C-13-NMR. Anal Bioanal Chem 409(1):307–315
He H, Sun Y, Li B, Yu Q, Wang T, Feng Y (2013) Boronate affinity solid-phase extraction based on functionalized magnesia-zirconia composite for enrichment of nucleosides in human urine. Anal Methods 5(6):1435–1441
Acknowledgements
The authors thank the financial support from the National Basic Research Program of China (973 Program) (2013CB910702), the National Natural Science Foundation of China (21475098, 2163506, 31670373).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1.76 mb)
Rights and permissions
About this article
Cite this article
Xu, J., Zhang, Z., He, XM. et al. Immobilization of zirconium-glycerolate nanowires on magnetic nanoparticles for extraction of urinary ribonucleosides. Microchim Acta 185, 43 (2018). https://doi.org/10.1007/s00604-017-2596-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2596-2