Abstract
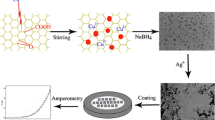
Simple and robustic mediator free graphene nanoflake modified glassy carbon electrode (GNF/GCE) was used for the simultaneous determination of vanillin (VAN) and guaiacol (GUA) in food products like chocolate and biscuits by using an amperometric method. Defect-free graphene nanoflakes were obtained by a surfactant assisted exfoliation approach. The resulting graphene flake was characterized by FE-SEM and Raman studies. The electrocatalytic oxidation of VAN and GUA occured at +0.59 V and +0.48 V vs. Ag/AgCl at GNF/GCE, under optimum experimental condition. A well-defined peak potential window (~160 mV) for the oxidation of VAn and GUA were observed in the modified electrode. Calibration curves of VAN and GUA were attained in the ranges of 0.1 × 10–7 M to 53 × 10–6 M and 0.3 × 10–7 M to 46 × 10–6 M with correlation coefficient of 0.9966 and 0.9951, respectively. A greater sensitivity and the detection limit of VAN and GUA at 1.24 × 10–8 M and 0.98 × 10–8 M were obtained by using mediator and metal free modified electrode system in phosphate buffer medium. The present method is quite modest and robustic for the detection of VAN and GUA at a trace level.

Sensitive electrochemical method for the quantitative detection of vanillin and guaiacol as food additive using defect-free graphene nanoflakes. Well-resolved oxidation peak current was shown in both analytes by differential pulse voltammetry method. The present method can be used for the simultaneous detection of vanillin and guaiacol in food samples





Similar content being viewed by others
References
Walton N, Mayer MJ, Narbad A (2003) Vanillin. Phytochemistry 63:505–515. doi:10.1016/S0031-9422(03)00149-3
Yan W, Meng H, NanNan S, WeiBing H (2014) Electrochemical detection of guaiacol in bamboo juice based on the enhancement effect of RGO nanosheets. Anal Methods 6:2729–2735. doi:10.1039/c4ay00195h
Yang X, Zhang F, Hu Y et al (2014a) Gold nanoparticals doping graphene sheets nanocomposites sensitized screen-printed carbon electrode as a disposable platform for voltammetric determination of guaiacol in bamboo juice. Int J Electrochem Sci 9:5061–5072
Murakami Y, Hirata A, Ito S, Shoji M, Tanaka S, Yasui T, Machino M, Fujisawa S (2007) Re-evaluation of cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in macrophages stimulated with lipopolysaccharide. Anticancer Res 27:801–808
Cava-Roda RM, Taboada-Rodríguez A, Valverde-Franco MT, Marín-Iniesta F (2012) Antimicrobial activity of vanillin and mixtures with cinnamon and clove essential oils in controlling Listeria monocytogenes and Escherichia coli O157:H7 in milk. Food Bioprocess Technol 5:2120–2131. doi:10.1007/s11947-010-0484-4
Deng P, Xu Z, Zeng R, Ding C (2015a) Electrochemical behavior and voltammetric determination of vanillin based on an acetylene black paste electrode modified with graphene-polyvinylpyrrolidone composite film. Food Chem. doi:10.1016/j.foodchem.2015.02.035
Takahashi M, Sakamaki S, Fujita A (2013) Simultaneous analysis of guaiacol and vanillin in a vanilla extract by using high-performance liquid chromatography with electrochemical detection. Biosci Biotechnol Biochem 77:595–600. doi:10.1271/bbb.120835
Rind FMA, Mughal UR, Memon AH et al (2009) Spectrophotometric analysis of vanillin from natural and synthetic sources. Asian J Chem 21:2849–2856
Hingse SS, Digole SB, Annapure US (2014) Method development for simultaneous detection of ferulic acid and vanillin using high-performance thin layer chromatography. J Anal Sci Technol 5:21. doi:10.1186/s40543-014-0021-6
Watanabe T, Yamamoto A, Nagai S, Terabe S (1998) Micellar electrokinetic chromatography as an alternative to high-performance liquid chromatography for separation and determination of phenolic compounds in Japanese spirituous liquor. J Chromatogr A 793:409–413. doi:10.1016/S0021-9673(97)00931-X
Pollnitz AP, Pardon KH, Sykes M, Sefton MA (2004) The effects of sample preparation and gas chromatograph injection techniques on the accuracy of measuring guaiacol, 4-methylguaiacol and other volatile oak compounds in oak extracts by stable isotope dilution analyses. J Agric Food Chem 52:3244–3252. doi:10.1021/jf035380x
Perumal V, Hashim U, Gopinath SCB et al (2015) “spotted Nanoflowers”: gold-seeded zinc oxide Nanohybrid for selective bio-capture. Sci Rep 5:12231. doi:10.1038/srep12231
Haarindraprasad R, Hashim U, Gopinath SCB et al (2015) Low temperature annealed zinc oxide nanostructured thin film-based transducers: characterization for sensing applications. PLoS One 10:e0132755. doi:10.1371/journal.pone.0132755
Gopinath SCB, Perumal V, Kumaresan R et al (2016) Nanogapped impedimetric immunosensor for the detection of 16 kDa heat shock protein against mycobacterium tuberculosis. Microchim Acta. doi:10.1007/s00604-016-1911-7
Gan T, Shi Z, Deng Y et al (2014a) Morphology-dependent electrochemical sensing properties of manganese dioxide-graphene oxide hybrid for guaiacol and vanillin. Electrochim Acta 147:157–166. doi:10.1016/j.electacta.2014.09.116
Randviir EP, Banks CE (2014) The oxygen reduction reaction at graphene modified electrodes. Electroanalysis 26:76–83. doi:10.1002/elan.201300477
Brownson DAC, Kampouris DK, Banks CE (2012) Graphene electrochemistry: fundamental concepts through to prominent applications. Chem Soc Rev 41:6944. doi:10.1039/c2cs35105f
Wantz F, Banks CE, Compton RG (2005) Edge plane pyrolytic graphite electrodes for stripping voltammetry: a comparison with other carbon based electrodes. Electroanalysis 17:655–661. doi:10.1002/elan.200403148
Wu H, Lin Q, Batchelor-McAUley C et al (2016) Stochastic detection and characterisation of individual ferrocene derivative tagged graphene Nanoplatelets. Analyst. doi:10.1039/C5AN02550H
Petridis C, Konios D, Stylianakis MM et al (2016) Solution processed reduced graphene oxide electrodes for organic photovoltaics. Nanoscale Horiz. doi:10.1039/C5NH00089K
Huang C, Li C, Shi G (2012) Graphene based catalysts. Energy Environ Sci 5:8848. doi:10.1039/c2ee22238h
Prabakaran E, Pandian K (2015) Amperometric detection of Sudan I in red chili powder samples using Ag nanoparticles decorated graphene oxide modified glassy carbon electrode. Food Chem 166:198–205. doi:10.1016/j.foodchem.2014.05.143
Yang Y, Shi W, Zhang R et al (2016a) Electrochemical exfoliation of graphite into nitrogen-doped graphene in glycine solution and its energy storage properties. Electrochim Acta 204:100–107. doi:10.1016/j.electacta.2016.04.063
Ilnicka A, Lukaszewicz JP (2016) Nanoscale exfoliation of graphene sheets for manufacturing of 3D mesoporous structures. J Nanosci Nanotechnol 16:9997–10000. doi:10.1166/jnn.2016.12085
Yang W, Chen G, Shi Z, Liu CC, Zhang L, Xie G, Cheng M, Wang D, Yang R, Shi D, Watanabe K, Taniguchi T, Yao Y, Zhang Y, Zhang G (2013) Epitaxial growth of single-domain graphene on hexagonal boron nitride. Nat Mater 12: 792-797. doi: 10.1038/nmat3695.
Wang J, Chen L, Wu N et al (2016) Uniform graphene on liquid metal by chemical vapour deposition at reduced temperature. Carbon N Y 96:799–804. doi:10.1016/j.carbon.2015.10.015
Shukla A, Bhat SD, Pillai VK (2016) Simultaneous unzipping and sulfonation of multi-walled carbon nanotubes to sulfonated graphene nanoribbons for nanocomposite membranes in polymer electrolyte fuel cells. J Memb Sci. doi:10.1016/j.memsci.2016.08.019
Haar S, Bruna M, Lian JX et al (2016) Liquid-phase exfoliation of graphite into single and few layers graphene with α-functionalized alkanes. J Phys Chem Lett Accepted. doi:10.1021/acs.jpclett.6b01260
Du W, Jiang X, Zhu L (2013) From graphite to graphene: direct liquid-phase exfoliation of graphite to produce single- and few-layered pristine graphene. J Mater Chem A 1:10592. doi:10.1039/c3ta12212c
Chen L, Tang Y, Wang K et al (2011) Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem Commun 13:133–137. doi:10.1016/j.elecom.2010.11.033
Hernández CN, García MBG, Santos DH et al (2016) Aqueous UV-VIS spectroelectrochemical study of the voltammetric reduction of graphene oxide on screen-printed carbon electrodes. Electrochem Commun 64:65–68. doi:10.1016/j.elecom.2016.01.017
Niu L, Li M, Tao X et al (2013) Salt-assisted direct exfoliation of graphite into high-quality, large-size, few-layer graphene sheets. Nano 5:7202–7208. doi:10.1039/c3nr02173d
Liu SQ, Sun WH, Hu FT (2012) Graphene nano sheet-fabricated electrochemical sensor for the determination of dopamine in the presence of ascorbic acid using cetyltrimethylammonium bromide as the discriminating agent. Sensors Actuators B Chem 173:497–504. doi:10.1016/j.snb.2012.07.052
Keeley GP, McEvoy N, Nolan H et al (2012) Simultaneous electrochemical determination of dopamine and paracetamol based on thin pyrolytic carbon films. Anal Methods 4:2048. doi:10.1039/c2ay25156f
Mutyala S, Mathiyarasu J (2015) Preparation of graphene nanoflakes and its application for detection of hydrazine. Sensors Actuators B Chem 210:692–699. doi:10.1016/j.snb.2015.01.033
Keeley GP, O’Neill A, Holzinger M et al (2011) DMF-exfoliated graphene for electrochemical NADH detection. Phys Chem Chem Phys 13:7747–7750. doi:10.1039/c1cp20060g
Lu W, Liu S, Qin X et al (2012) High-yield, large-scale production of few-layer graphene flakes within seconds: using chlorosulfonic acid and H2O2 as exfoliating agents. J Mater Chem 22:8775. doi:10.1039/c2jm16741g
Wu C, Cheng Q, Wu K (2015) Electrochemical functionalization of N -methyl-2-pyrrolidone-exfoliated graphene nanosheets as highly sensitive analytical platform for phenols. Anal Chem 87:3294–3299. doi:10.1021/ac504309j
Lu J, Drzal LT, Worden RM, Lee I (2007) Simple fabrication of a highly sensitive glucose biosensor using enzymes immobilized in exfoliated graphite nanoplatelets nafion membrane. Chem Mater 19:6240–6246. doi:10.1021/cm702133u
Song X, Shi Z, Tan X et al (2014) One-step solvent exfoliation of graphite to produce a highly-sensitive electrochemical sensor for tartrazine. Sensors Actuators B Chem 197:104–108. doi:10.1016/j.snb.2014.02.064
Yang X, Long J, Sun D (2016b) Highly-sensitive electrochemical determination of rutin using NMP-exfoliated graphene Nanosheets-modified electrode. Electroanalysis 28:83–87. doi:10.1002/elan.201500449
Shahriary L, Athawale A (2014) Graphene oxide synthesized by using modified hummers approach. Int J Renew Energy Environ Eng 2:58–63
Bard AJ, Faulkner LR (2000) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Shang L, Zhao F, Zeng B (2014) Sensitive voltammetric determination of vanillin with an AuPd nanoparticles-graphene composite modified electrode. Food Chem 151:53–57. doi:10.1016/j.foodchem.2013.11.044
Peng J, Hou C, Hu X (2012) A graphene-based electrochemical sensor for sensitive detection of vanillin. Int J Electrochem Sci 7:1724–1733
Laviron E (1972) Theoretical study of a reversible reaction followed by a chemical reaction in thin layer linear potential sweep voltammetry. J Electroanal Chem Interfacial Electrochem 39:1–23. doi:10.1016/S0022-0728(72)80472-8
Gan T, Shi Z, Deng Y, Sun J, Wang H (2014b) Morphology dependent electrochemical sensing properties of manganese dioxide graphene oxide hybrid for guaiacol and vanillin. Electrochim Acta 147:157–166. doi:10.1016/j.electacta.2014.09.116
Yang X, Zhang F, Yangjian H, Dizhao C, Zegiang H, Xiong L (2014b) Gold nanoparticles doping graphene sheets nanocomposites sensitized screen-printed carbon electrode as a disposable platform for Voltammetric determination of Guaiacol in bamboo juice. Int J Electrochem Sci 9:5061–5072
Gan T, Zhaoxia S, Yaping D, Junyong S, Haibo W (2014c) Morphology-dependent electrochemical sensing properties of manganese dioxide-graphene oxide hybrid for guaiacol and vanillin. Electrochim Acta 147:157–166
Yong Sun J, Gan T, Deng Y P, Shi Z X, Zhen L (2015) Pt nanoparticles - functionalized Hierarchically porous - Al2O3 hallow spheres based electrochemical sensor for ultrasensitive Guaiacol Detection. Sensors and Actuators B 211:339–345.doi:org/10.1016/j.snb.2015.01.104
Chawla S, Rawal R, Pundir CS (2011) Fabrication of polyphenol biosensor based on laccase immobilized on copper nanoparticles/chitosan/multiwalled carbon nanotube/polyaniline-modified gold electrode. J Biotechnol 156:39–45. doi:10.1016/j.jbiotech.2011.08.008
Brondani D, Soura BD, Souza S, Neves A, Vieira IC (2013) PEI-coated gold nanoparticles decorated with laccase: a new platform for direct electrochemistry of enzymes and biosensing applications. Biosens Bioelectron 42:242–247. doi:10.1016/j.bios.2012.10.087
Chawla S, Rawal R, Kumar D, Pundir CS (2012) Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold elecrode. Anal Biochem 430:16–23
Wu Y, Huang M, Song N, Hu W (2014) Electrochemical detection of guaiacol in bamboo juice based on the enhancement effect of RGO nanosheets. Anal Methods 6:2729–2735. doi:10.1039/c4ay00195h
Yang W, Wu L, Zhao F, Zen B (2017) A vanillin electrochemical sensor based on molecularly imprinted poly (1-vinyl-3-octylimidazole hexafluoride phosphorus) multiwalled carbon nanotubes@polydopamine-carboxyl single walled carbon nanotubes composite. Sensors Actuators B 239:481–487. doi:10.1016/j.snb.2016.08.041
Vilan ATE, Kwak CH, Hwang SK, Huh YS, Ahn WS, Han YK (2016) Fabrication of palladium nanoparticles on porous aromatic frameworks as a sensing platform to detect vanillin. Appl Mater Interfaces 8:12740–12747. doi:10.1021/acsami.6b03942
Ziyatdinova G, Kozlova E, Ziganshina E, Budnikov H (2016) Surfactant/carbon nanofibrous modified electrode for the determination of vanillin. Monatsh Chem 147:191–200. doi:10.1007/s00706-015-1559-8
Veeramani V, Madhu R, Chen SM, Veerakumar P, Syu JJ, Liu SB (2016) Cajeput tree bark derived activated carbon for the practical electrochemical detection of vanillin. New J Chem 39:9109–9115. doi:10.1039/C5NJ01634G
Silva TR, Brondani D, Zapp E, Vieira IC (2015) Electrochemical sensor based on gold nanoparticles stabilized in poly (allylamine hydrochloride) for determination of vanillin. Electroanalysis 27:465–472. doi:10.1002/elan.201400517
Acknowledgements
One of author (Dr.K.P.) is grateful to Prof. M.S. Ramachandra Rao, Department of Physics, IIT Madras for providing Raman Instrument. And also thankful to the Prof. M. V. Sangaranarayanan and M. V. Beena, Department of Chemistry, IIT Madras for providing EIS studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 560 kb)
Rights and permissions
About this article
Cite this article
Kalaiyarasi, J., Meenakshi, S., Pandian, K. et al. Simultaneous voltammetric determination of vanillin and guaiacol in food products on defect free graphene nanoflakes modified glassy carbon electrode. Microchim Acta 184, 2131–2140 (2017). https://doi.org/10.1007/s00604-017-2161-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2161-z