Abstract
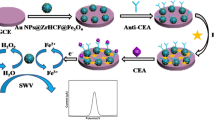
A reagentless and label-free voltammetric modified electrode was developed for the carcinoembryonic antigen (CEA), an important biomarker for colorectal adenocarcinoma. It is based on the use of a reticular hybrid composite consisting of polyaniline (PANI) nanowires grown on the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) that was doped with an ionic liquid (IL). The composite features excellent electrical conductivity and a porous structure, a high specific surface and inherent redox activity. It was placed on a glassy carbon electrode (GCE) and antibody against CEA was immobilized on its surface. The redox current of PANI, measured typically at 0.16 V (vs. Ag/AgCl) serves as the analytical information. This electrode displays linear range that extends from 0.001 to 10 ng mL−1, with a detection limit as low as 0.7 pg mL−1. It also possesses excellent temporal stability and selectivity.

A sensitive, label-free and reagentless modified electrode for CEA was developed based on the combination of two different conducting polymers, polyaniline nanowires and porous PEDOT/IL nanocomposite.






Similar content being viewed by others
References
Lu W, Cao X, Tao L, Ge J, Dong J, Qian W (2014) A novel label-free amperometric immunosensor for carcinoembryonic antigen based on Ag nanoparticle decorated infinite coordination polymer fibres. Biosens Bioelectron 57:219–225
Iwazawa T, Kanoh T, Matsui S, Monden T (2000) Diagnosis of lung cancer metastasis with CEA extracted from the dissected regional lymph nodes. Lung Cancer 29(1):254–254
Miao J, Wang X, Lu L, Zhu P, Mao C, Zhao H, Song Y, Shen J (2014) Electrochemical immunosensor based on hyperbranched structure for carcinoembryonic antigen detection. Biosens Bioelectron 58:9–16
Zamcheck N, Martin EW (1981) Factors controlling the circulating CEA levels in pancreatic cancer: some clinical correlations. Cancer 47:1620–1627
Li BT, Lou E, Hsu M, Helena AY, Naidoo J, Zauderer MG, Sima C, Johnson ML, Daras M, DeAngelis LM (2016) Serum biomarkers associated with clinical outcomes fail to predict brain metastases in patients with stage IV non-small cell lung cancers. PLoS One 11:e0146063
Yuan J, Wang G, Majima K, Matsumoto K (2001) Synthesis of a terbium fluorescent chelate and its application to time-resolved fluoroimmunoassay. Anal Chem 73:1869–1876
Fu Z, Yang Z, Tang J, Liu H, Yan F, Ju H (2007) Channel and substrate zone two-dimensional resolution for chemiluminescent multiplex immunoassay. Anal Chem 79:7376–7382
Sun X, Lei C, Guo L, Zhou Y (2016) Giant magneto-resistance based immunoassay for the tumor marker carcinoembryonic antigen. Microchim Acta 183(3):1107–1114
Cioffi M, Vietri M, Gazzerro P, Magnetta R, D’Auria A, Durante A, Nola E, Puca G, Molinari A (2001) Serum anti-p53 antibodies in lung cancer: comparison with established tumor markers. Lung Cancer 33:163–169
Gao X, Zhang Y, Chen H, Chen Z, Lin X (2011) Amperometric immunosensor for carcinoembryonic antigen detection with carbon nanotube-based film decorated with gold nanoclusters. Anal Biochem 414:70–76
Huang J, Tian J, Zhao Y, Zhao S (2015) Ag/Au nanoparticles coated graphene electrochemical sensor for ultrasensitive analysis of carcinoembryonic antigen in clinical immunoassay. Sensors Actuators B Chem 206:570–576
Liu J, Wang J, Wang T, Li D, Xi F, Wang J, Wang E (2015) Three-dimensional electrochemical immunosensor for sensitive detection of carcinoembryonic antigen based on monolithic and macroporous graphene foam. Biosens Bioelectron 65:281–286
Lin J, Zhang H, Niu S (2015) Simultaneous determination of carcinoembryonic antigen and α-fetoprotein using an ITO immunoelectrode modified with gold nanoparticles and mesoporous silica. Microchim Acta 182(3–4):719–726
Xu T, Li X, Xie Z, Li X, Zhang H (2015) Poly (o-phenylenediamine) nanosphere-conjugated capture antibody immobilized on a glassy carbon electrode for electrochemical immunoassay of carcinoembryonic antigen. Microchim Acta 182(15–16):2541–2549
Zhao D, Wang Y, Nie G (2016) Electrochemical immunosensor for the carcinoembryonic antigen based on a nanocomposite consisting of reduced graphene oxide, gold nanoparticles and poly (indole-6-carboxylic acid). Microchim Acta 183(11):2925–2932
Chen J, Yan F, Dai Z, Ju H (2005) Reagentless amperometric immunosensor for human chorionic gonadotrophin based on direct electrochemistry of horseradish peroxidase. Biosens Bioelectron 21:330–336
Kang Y, Kim SK, Lee C (2004) Doping of polyaniline by thermal acid-base exchange reaction. Mater Sci Eng C 24:39–41
Heeger AJ (2001) Semiconducting and metallic polymers: the fourth generation of polymeric materials. J Phys Chem B 105:8475–8491
Luo YC, Do JS (2004) Urea biosensor based on PANi(urease)-Nafion®/Au composite electrode. Biosens Bioelectron 20:15–23
Colleran JJ, Breslin CB (2012) Simultaneous electrochemical detection of the catecholamines and ascorbic acid at PEDOT/S-β-CD modified gold electrodes. J Electroanal Chem 667:30–37
Kannan B, Williams D, Laslau EC, Travas-Sejdic J (2012) The electrochemical growth of highly conductive single PEDOT (conducting polymer):BMIPF6 (ionic liquid) nanowires. J Mater Chem 22:18132–18135
Xu G, Li B, Wang X, Luo X (2014) Electrochemical sensor for nitrobenzene based on carbon paste electrode modified with a poly(3,4-ethylenedioxythiophene) and carbon nanotube nanocomposite. Microchim Acta 181:463–469
Kakhki S, Barsan MM, Shams E, Brett CM (2012) Development and characterization of poly(3,4-ethylenedioxythiophene)-coated poly(methylene blue)-modified carbon electrodes. Synth Met 161:2718–2726
Tsuda T, Hussey CL (2007) Electrochemical applications of room-temperature ionic liquids. Electrochem Soc Interface 16:42–49
Kwak K, Kumar SS, Pyo K, Lee D (2013) Ionic liquid of a gold nanocluster: a versatile matrix for electrochemical biosensors. ACS Nano 8:671–679
Quinn BM, Ding Z, Moulton R, Bard AJ (2002) Novel electrochemical studies of ionic liquids. Langmuir 18:1734–1742
Badre C, Marquant L, Alsayed AM, Hough LA (2012) Highly conductive poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) films using 1-ethyl-3-methylimidazolium tetracyanoborate ionic liquid. Adv Funct Mater 22:2723–2727
Pringle JM, Lynam C, Wallace GG, Forsyth M, MacFarlane DR (2008) One-step synthesis of conducting polymer-noble metal nanoparticle composites using an ionic liquid. Adv Funct Mater 18:2031–2040
Sheng G, Xu G, Xu S, Wang S, Luo X (2015) Cost-effective preparation and sensing application of conducting polymer PEDOT/ionic liquid nanocomposite with excellent electrochemical properties. RSC Adv 5:20741–20746
Adeloju SB, Wallace GG (1996) Conducting polymers and the bioanalytical sciences: new tools for biomolecular communications. Analyst 121(6):699–703
Lassalle N, Vieil E, Correia JP, Abrantes LM (2001) Study of DNA hybridization by photocurrent spectroscopy. Synth Met 119(1–3):407–408
Livache T, Roget A, Dejean E, Barthet C, Bidan G, Teoulen R (1995) Biosensing effects in functionalized electroconducting conjugated polymer layers: addressable DNA matrix for the detection of gene mutations. Synth Met 71(1):2143–2146
Jia Q, Li J, Wang L, Zhu G, Zheng M (2007) Electrically conductive epoxy resin composites containing polyaniline with different morphologies. Mater Sci Eng A 448(1):356–360
Wang Z, Liu S, Wu P, Cai C (2009) Detection of glucose based on direct electron transfer reaction of glucose oxidase immobilized on highly ordered polyaniline nanotubes. Anal Chem 81(4):1638–1645
Peng H, Zhang L, Soeller C, Travas-Sejdic J (2009) Conducting polymers for electrochemical DNA sensing. Biomater 30(11):2132–2148
Peng H, Soeller C, Vigar NA, Caprio V, Travas-Sejdic J (2007) Label-free detection of DNA hybridization based on a novel functionalized conducting polymer. Biosens Bioelectron 22(9):1868–1873
Li Y, Zhang Q, Zhao X, Yu P, Wu L, Chen D (2012) Enhanced electrochemical performance of polyaniline/sulfonated polyhedral oligosilsesquioxane nanocomposites with porous and ordered hierarchical nanostructure. J Mater Chem 22:1884–1892
Li Y, Zhao X, Yu P, Zhang Q (2012) Oriented arrays of polyaniline nanorods grown on graphite nanosheets for an electrochemical supercapacitor. Langmuir 29:493–500
Limbut W, Kanatharana P, Mattiasson B, Asawatreratanakul P, Thavarungkul P (2006) A reusable capacitive immunosensor for carcinoembryonic antigen (CEA) detection using thiourea modified gold electrode. Anal Chim Acta 56:55–61
Kimura H, Matsuzawa S, Tu CY, Kitamori T, Sawada T (1996) Ultrasensitive heterogeneous immunoassay using photothermal deflection spectroscopy. 2. Quantitation of ultratrace carcinoembryonic antigen in human sera. Anal Chem 68:3063–3067
Laboria N, Fragoso A, Kemmner W, Latta D, Nilsson O, Luz Botero M, Drese K, O’Sullivan CK (2010) Amperometric immunosensor for carcinoembryonic antigen in colon cancer samples based on monolayers of dendritic bipodal scaffolds. Anal Chem 82:1712–1719
Norouzi P, Gupta VK, Faridbod F, Pirali-Hamedani M, Larijani B, Ganjali MR (2011) Carcinoembryonic antigen admittance biosensor based on Au and ZnO nanoparticles using FFT admittance voltammetry. Anal Chem 83:1564–1570
Tang D, Yuan R, Chai Y (2006) Magnetic core-shell Fe3O4@Ag nanoparticles coated carbon paste interface for studies of carcinoembryonic antigen in clinical immunoassay. J Phys Chem B 110:11640–11646
Yang M, Chen Y, Xiang Y, Yuan R, Chai Y (2013) In situ energy transfer quenching of quantum dot electrochemiluminescence for sensitive detection of cancer biomarkers. Biosens Bioelectron 50:393–398
Zhang Y, Lu F, Yan Z, Wu D, Ma H, Du B, Wei Q (2015) Electrochemiluminescence immunosensing strategy based on the use of Au@Ag nanorods as a peroxidase mimic and NH4CoPO4 as a supercapacitive supporter: application to the determination of carcinoembryonic antigen. Microchim Acta 182(7–8):1421–1429
Lu W, Lin T, Wang Y, Cao X, Dong J, Qian W (2015) An electrochemical immunosensor for simultaneous multiplexed detection of two lung cancer biomarkers using Au nanoparticles coated resin microspheres composed of L-tryptophan and caffeic acid. Ionics 21:1141–1152
Guo A, Li Y, Cao W, Meng X, Wu D, Wei Q, Du B (2015) An electrochemical immunosensor for ultrasensitive detection of carbohydrate antigen 199 based on Au@CuxOS yolk-shell nanostructures with porous shells as labels. Biosens Bioelectron 63:39–46
Acknowledgements
This research was supported by the National Natural Science Foundation of China (21275087, 21422504), the Natural Science Foundation of Shandong Province of China (JQ201406 and ZR2016BM05), and the Taishan Scholar Program of Shandong Province of China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 135 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Hui, N. & Luo, X. Reagentless and label-free voltammetric immunosensor for carcinoembryonic antigen based on polyaniline nanowires grown on porous conducting polymer composite. Microchim Acta 184, 889–896 (2017). https://doi.org/10.1007/s00604-016-2068-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-2068-0