Abstract
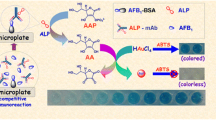
The authors report on a competitive potentiometric immunoassay for aflatoxin B1 (AFB1) in food that displays distinctly improved sensitivity. Gold nanoparticles (AuNPs; 16 nm i. d.) were functionalized with polyclonal anti-AFB1 antibody (pAb), whilst the sensor electrode was prepared by immobilizing AFB1-bovine serum albumin conjugate (AFB1-BSA) on a glassy carbon electrode. Upon addition of target AFB1, competitive immunobinding occurs between the analyte and AFB1-BSA for the labeled pAb on the AuNPs. The change in the surface charge as a result of the antigen-antibody reaction causes a shift in the electrical potential. With increasing concentrations of analyte (AFB1), the quantity of pAb-AuNP captured by the electrode decreases. The shift in the output potential is linearly proportional to the logarithm of AFB1 concentration in the 0.1 to 5.0 μg · kg−1 range, with a detection limit (LOD) of 87 ng · kg−1 (87 ppt). An intermediate precision of 10.9 % was accomplished in batch-to-batch identification. The selectivity over AFB2 with similar chemical structure is acceptable. The method accuracy was evaluated by analyzing naturally contaminated and spiked peanut samples, giving consistent results (with RSD values of <12 %) between this immunoassay and the commercial ELISA.

A potentiometric immunosensor was designed for detection of AFB1 by using nanogold-labeled antibodies as the signal-amplification tags.




Similar content being viewed by others
References
Tarasov A, Gray D, Tsai M, Shields N, Montrose A, Creedon N, Lovera P, O’Riordan A, Mooney M, Vogel E (2016) A potentiometric biosensor for rapid on-site disease diagnostics. Biosens Bioelectron 79:669–678
Arida H (2015) Novel pH microsensor based on a thin film gold electrode modified with lead dioxide nanoparticles. Microchim Acta 182:149–156
Zhang Q, Prabhu A, San A, Al-Sharab J, Levon K (2015) A polyaniline based ultrasensitive potentiometric immunosensor for cardiac troponin complex detection. Biosens Bioelectron 72:100–106
Ibupoto Z, Khun K, Liu X, Willander M (2014) A potentiometric biosensor for the detection of notch 3 using functionalized ZnO nanorods. J Nanosci Nanotechnol 14:6704–6710
Feng C, Xu Y, Song M (2000) Study on highly sensitive potentiometric IgG immunosensor. Sens Actuators B 66:190–192
Piao M, Noh H, Rahman M, Won M, Shim Y (2008) Label-free detection of bisphenol A using a potentiometric immunosensor. Electroanalysis 20:30–37
Zhang B, Liu B, Chen G, Tang D (2014) Competitive-type displacement reaction for direct potentiometric detection of low-abundance protein. Biosens Bioelectron 53:465–471
Tang D, Tang J, Su B, Ren J, Chen G (2010) Simultaneous determination of five-type hepatitis virus antigens in 5 min using an integrated automatic electrochemical immunosensor array. Biosens Bioelectron 25:1658–1662
Tang D, Yuan R, Chai Y (2007) Magnetic control of an electrochemical microfluidic device with an arrayed immunosensor for simultaneous multiple immunoassays. Clin Chem 53:1323–1329
Mleczko J, Defort A, Koziol J, Nguyen T, Mironczyk A, Zapotoczny B, Nowak-Jary J, Gronczewska E, Marc M, Dudek M (2016) Limitation of tuning the antibody-antigen reaction by changing the value of pH and its consequence for hyperthermia. J Biotechnol 159:421–427
Kim D, Bong J, Yoo G, Chang S, Park M, Chang Y, Kang M, Jose J, Pyun J (2016) Microbead-based immunoassay using the outer membrane layer of Escherichia coli combined with autodisplayed Z-domains. Appl Surf Sci 362:146–153
Ding J, Wang X, Qin W (2013) Pulsed galvanostatic control of a polymeric membrane ion-selective electrode for potentiometric immunoassays. ACS Appl Mater Interfaces 5:9488–9493
Rameil S, Schubert P, Grundmann P, Dietrich R, Martlbauer E (2010) Use of 3-(4-hydroxyphenyl) propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal Chim Acta 661:122–127
Wang X, Niessner R, Tang D, Knopp D (2016) Nanoparticle-based immunosensors and immunoassays for aflatoxins. Anal Chim Acta 912:10–23
Korotcenkov G, Brinzari V, Cho B (2016) Conductometric gas sensors based on metal oxides modified with gold nanoparticles: a review. Microchim Acta 183:1033–1054
Yusoff N, Pandikumar A, Ramaraj R, Lim H, Huang N (2015) Gold nanoparticle based optical and electrochemical sensing of dopamine. Microchim Acta 182:2091–2114
Luan Y, Chen J, Xie G, Li G, Ping H, Ma Z, Lua A (2015) Visual and microplate detection of aflatoxin B2 based on NaCl-induced aggregation of aptamer-modified gold nanoparticles. Microchim Acta 182:995–1001
Arduini F, Amine A, Moscone D, Palleschi G (2010) Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection. Microchim Acta 170:193–214
Tang D, Liu Y, Zhou Q, Lin Y, Li P, Niessner R, Knopp D (2014) Low-cost and highly sensitive immunosensing platform for aflatoxins using one-step competitive displacement reaction mode and portable glucometer-based detection. Anal Chem 86:11451–11458
Zhang B, Liu B, Tang D, Niessner R, Chen G, Knopp D (2012) DNA-based hybridization chain reaction for amplified bioelectronic signal and ultrasensitive detection of proteins. Anal Chem 84:5392–5399
Chen Z, Ying S, Liu J, Zhan P, Zhao Y (2016) PRiME pass-through cleanup for the fast determination of aflatoxins in human serum by using LC-MS/MS. Anal Methods 8:1457–1462
Joshi S, Segarra-Fas A, Peters J, Zuihof H, Beek T, Nielen W (2016) Multiplex surface plasmon resonance biosensing and its transferability towards imaging nanoplasmonics for detection of mycotoxins in barley. Analyst 141:1307–1318
Wang B, Chen Y, Wu Y, Weng B, Liu Y, Lu Z, Li C, Yu C (2016) Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB1. Biosens Bioelectron 78:23–30
Jettanajit A, Nhujak T (2016) Determination of mycotoxins in brown rice using QuEChERS sample preparation and UHPLC-MS-MS. J Chromatogr Sci 54:720–729
Ma H, Sun J, Zhang Y, Bian C, Xia S, Zhen T (2016) Label-free immunosensor based on one-step electrodeposition of chitosan-gold nanoparticles biocompatible film on Au microelectrode for determination of aflatoxin B1 in maize. Biosens Bioelectron 80:222–229
Sun C, Li H, Koidis A, Chen Q (2016) Quantifying aflatoxin B1 in peanut oil using fabricating fluorescence probes based on upconversion nanoparticles. Spectrochim Acta A 165:120–126
Sun Q, Zhu Z, Deng Q, Liu J, Shi G (2016) A “green” method to detect aflatoxin B1 residue in plant oil based on a colloidal gold immunochromatographic assay. Anal Methods 8:564–569
Liao J, Li H (2010) Lateral flow immunodipstick for visual detection of aflatoxin B1 in food using immuno-nanoparticles composed of a silver core and a gold shell. Microchim Acta 171:289–295
Acknowledgments
Support by the National Natural Science Foundation of China (41176079 & 21475025), the Research Fund for the Doctoral Program of Hubei University for Nationalities (MY2014B014), the National Science Foundation of Fujian Province (2014J07001), and the Program for Changjiang Scholars and Innovative Research Team in University (IRT1116) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
(DOC 197 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Lv, S., Lu, M. et al. Potentiometric competitive immunoassay for determination of aflatoxin B1 in food by using antibody-labeled gold nanoparticles. Microchim Acta 183, 2815–2822 (2016). https://doi.org/10.1007/s00604-016-1929-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1929-x