Abstract
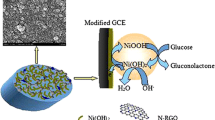
The authors describe a non-enzymatic amperometric sensor for glucose that was prepared by electrodeposition of cobalt oxide nanoparticles on a graphene paste that is readily obtained by mixing reduced graphene oxide (r-GO) nanosheets and mineral oil in a mortar. An electrode was prepared and characterized by cyclic voltammetry and electrochemical impedance spectroscopy. Cobalt oxide nanoparticles were electrochemically deposited on the electrode, and their morphology was characterized by field emission scanning electron microscopy. Cyclic voltammetric and chronoamperometric studies showed the modified electrode to display electrocatalytic activity toward the direct oxidation of glucose, best at a working potential of 0.45 V (vs. SCE) in 0.1 M NaOH solution. The sensor exhibits a linear response in the 40 to 4000 μM glucose concentration range, has a detection limit as low as 1.4 μM (at an S/N ratio of 3), and a sensitivity of 1.21 μA∙μM−1∙cm−2. The electrode was successfully applied to the determination of glucose in (spiked) human serum where it showed recoveries between 97.2 and 103.4 %.

A non-enzymatic amperometric sensor for determination of glucose level in serum samples was prepared by electrodeposition of cobalt oxide nanoparticles on a graphene paste electrode.





Similar content being viewed by others
References
Wang J (2008) Electrochemical glucose biosensors. Chem Rev 108(2):814–825. doi:10.1021/cr068123a
Wang G, He X, Wang L, Gu A, Huang Y, Fang B, Geng B, Zhang X (2012) Non-enzymatic electrochemical sensing of glucose. Microchim Acta 180(3):161–186. doi:10.1007/s00604-012-0923-1
Harms D, Meyer J, Westerheide L, Krebs B, Karst U (1999) Determination of glucose in soft drinks using its enzymatic oxidation and the detection of formed hydrogen peroxide with a dinuclear iron(III) complex. Anal Chim Acta 401(1–2):83–90. doi:10.1016/S0003-2670(99)00514-0
Ci S, Huang T, Wen Z, Cui S, Mao S, Steeber DA, Chen J (2014) Nickel oxide hollow microsphere for non-enzyme glucose detection. Biosensors Bioelectron 54(0):251–257. doi:10.1016/j.bios.2013.11.006
Yu H, Jin J, Jian X, Wang Y, G-c Q (2013) Preparation of cobalt oxide nanoclusters/overoxidized polypyrrole composite film modified electrode and its application in nonenzymatic glucose sensing. Eectroanal 25(7):1665–1674. doi:10.1002/elan.201300035
Wang L, Lu X, Ye Y, Sun L, Song Y (2013) Nickel-cobalt nanostructures coated reduced graphene oxide nanocomposite electrode for nonenzymatic glucose biosensing. Electrochim Acta 114:484–493. doi:10.1016/j.electacta.2013.10.125
Chen X, Wu G, Cai Z, Oyama M, Chen X (2013) Advances in enzyme-free electrochemical sensors for hydrogen peroxide, glucose, and uric acid. Microchim Acta 181(7):689–705. doi:10.1007/s00604-013-1098-0
Zhou X, Zheng X, Lv R, Kong D, Li Q (2013) Electrodeposition of platinum on poly(glutamic acid) modified glassy carbon electrode for non-enzymatic amperometric glucose detection. Electrochim Acta 107:164–169. doi:10.1016/j.electacta.2013.05.146
Chen X, Tian X, Zhao L, Huang Z, Oyama M (2013) Nonenzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with graphene nanosheets and Pt-Pd bimetallic nanocubes. Microchim Acta 181(7):783–789. doi:10.1007/s00604-013-1142-0
Ding Y, Wang Y, Su L, Bellagamba M, Zhang H, Lei Y (2010) Electrospun Co3O4 nanofibers for sensitive and selective glucose detection. Biosensors Bioelectron 26(2):542–548. doi:10.1016/j.bios.2010.07.050
Karuppiah C, Palanisamy S, Chen S-M, Veeramani V, Periakaruppan P (2014) A novel enzymatic glucose biosensor and sensitive non-enzymatic hydrogen peroxide sensor based on graphene and cobalt oxide nanoparticles composite modified glassy carbon electrode. Sensors Actuators B Chem 196(0):450–456. doi:10.1016/j.snb.2014.02.034
Zhong A, Luo X, Chen L, Wei S, Liang Y, Li X (2014) Enzyme-free sensing of glucose on a copper electrode modified with nickel nanoparticles and multiwalled carbon nanotubes. Microchim Acta 182(5):1197–1204. doi:10.1007/s00604-014-1443-y
Dhara K, Thiagarajan R, Nair BG, Thekkedath GSB (2015) Highly sensitive and wide-range nonenzymatic disposable glucose sensor based on a screen printed carbon electrode modified with reduced graphene oxide and Pd-CuO nanoparticles. Microchim Acta 182(13):2183–2192. doi:10.1007/s00604-015-1549-x
Khan SB, Karimov KS, Chani MTS, Asiri AM, Akhtar K, Fatima N (2015) Impedimetric sensing of humidity and temperature using CeO2–Co3O4 nanoparticles in polymer hosts. Microchim Acta 182(11):2019–2026. doi:10.1007/s00604-015-1529-1
Shinde VR, Mahadik SB, Gujar TP, Lokhande CD (2006) Supercapacitive cobalt oxide (Co3O4) thin films by spray pyrolysis. Appl Surf Sci 252(20):7487–7492. doi:10.1016/j.apsusc.2005.09.004
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183–191
Zhang Y-H, Chen Y-B, Zhou K-G, Liu C-H, Zeng J, Zhang H-L, Peng Y (2009) Improving gas sensing properties of graphene by introducing dopants and defects: a first-principles study. Nanotechnology 20(18):185504
Gutierrez F, Comba FN, Gasnier A, Gutierrez A, Galicia L, Parrado C, Rubianes MD, Rivas GA (2014) Graphene paste electrode: analytical applications for the quantification of dopamine, phenolic compounds and ethanol. Eectroanal 26(8):1694–1701. doi:10.1002/elan.201400247
Parvin MH (2011) Graphene paste electrode for detection of chlorpromazine. Electrochem Commun 13(4):366–369. doi:10.1016/j.elecom.2011.01.027
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339. doi:10.1021/ja01539a017
Kovtyukhova NI, Ollivier PJ, Martin BR, Mallouk TE, Chizhik SA, Buzaneva EV, Gorchinskiy AD (1999) Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem Mater 11(3):771–778. doi:10.1021/cm981085u
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y, Wu Y, Nguyen ST, Ruoff RS (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565. doi:10.1016/j.carbon.2007.02.034
Chen L, Tang Y, Wang K, Liu C, Luo S (2011) Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem Commun 13(2):133–137. doi:10.1016/j.elecom.2010.11.033
Barbero C, Planes GA, Miras MC (2001) Redox coupled ion exchange in cobalt oxide films. Electrochem Commun 3(3):113–116. doi:10.1016/S1388-2481(01)00107-2
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101(1):19–28. doi:10.1016/S0022-0728(79)80075-3
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications, vol 2. Wiley, New York
Yang J, Zhang W-d, Gunasekaran S (2011) A low-potential, H2O2-assisted electrodeposition of cobalt oxide/hydroxide nanostructures onto vertically-aligned multi-walled carbon nanotube arrays for glucose sensing. Electrochim Acta 56(16):5538–5544. doi:10.1016/j.electacta.2011.03.087
Buratti S, Brunetti B, Mannino S (2008) Amperometric detection of carbohydrates and thiols by using a glassy carbon electrode coated with Co oxide/multi-wall carbon nanotubes catalytic system. Talanta 76(2):454–457. doi:10.1016/j.talanta.2008.03.031
Li S-J, Du J-M, Chen J, Mao N-N, Zhang M-J, Pang H (2013) Electrodeposition of cobalt oxide nanoparticles on reduced graphene oxide: a two-dimensional hybrid for enzyme-free glucose sensing. J Solid State Electrochem 18(4):1049–1056. doi:10.1007/s10008-013-2354-2
Dong X-C, Xu H, Wang X-W, Huang Y-X, Chan-Park MB, Zhang H, Wang L-H, Huang W, Chen P (2012) 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS Nano 6(4):3206–3213. doi:10.1021/nn300097q
Wang X, Dong X, Wen Y, Li C, Xiong Q, Chen P (2012) A graphene-cobalt oxide based needle electrode for non-enzymatic glucose detection in micro-droplets. Chem Commun 48(52):6490–6492. doi:10.1039/C2CC32674D
Meng F, Shi W, Sun Y, Zhu X, Wu G, Ruan C, Liu X, Ge D (2013) Nonenzymatic biosensor based on CuxO nanoparticles deposited on polypyrrole nanowires for improving detectionrange. Biosensors Bioelectron 42:141–147. doi:10.1016/j.bios.2012.10.051
Nayak P, Nair SP, Ramaprabhu S (2015) Enzyme-less and low-potential sensing of glucose using a glassy carbon electrode modified with palladium nanoparticles deposited on graphene-wrapped carbon nanotubes. Microchimica Acta: 1–8. doi:10.1007/s00604-015-1729-8
Mei H, Wu W, Yu B, Li Y, Wu H, Wang S, Xia Q (2015) Non-enzymatic sensing of glucose at neutral pH values using a glassy carbon electrode modified with carbon supported Co@Pt core-shell nanoparticles. Microchim Acta 182(11):1869–1875. doi:10.1007/s00604-015-1524-6
Roh S, Kim J (2014) Electrodeposition of three-dimensionally assembled platinum spheres on a gold-coated silicon wafer, and its application to nonenzymatic sensing of glucose. Microchim Acta 182(3):849–854. doi:10.1007/s00604-014-1397-0
Acknowledgments
The authors gratefully acknowledge the research council of Azarbaijan Shahid Madani University for financial support (Project No. 217/d/12045).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 71 kb)
Rights and permissions
About this article
Cite this article
Heidari, H., Habibi, E. Amperometric enzyme-free glucose sensor based on the use of a reduced graphene oxide paste electrode modified with electrodeposited cobalt oxide nanoparticles. Microchim Acta 183, 2259–2266 (2016). https://doi.org/10.1007/s00604-016-1862-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1862-z