Abstract
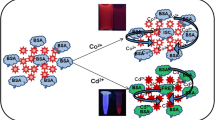
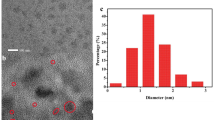
A one-pot route has been developed for the preparation of bovine serum albumin-templated nickel-doped bimetallic gold-nickel nanoclusters (BSA-Au-Ni NCs) at a 10:1 M ratio of the precursor salts in a BSA matrix under alkaline conditions. The metal ions are reduced to the metal alloys by BSA. The resulting NCs display strong fluorescence and dual emission with peaks at 405 and 640 nm, respectively, under excitation at 340 nm. Fluorescence is strongly enhanced on addition of Cd(II) ions, but quenched on addition of Hg(II) ions. The findings have been exploited to design a fluorometric method for the separate determination of Cd(II) and Hg(II), respectively. The optimized analytical nanosystem displays relatively good dynamics between enhancement and quenching. Cd(II) and Hg(II) can be quantified in the 0 to 200 and 0 nM to 24 μM, respectively. The limits of detection are ~1.8 nM in both cases, which indicates the highest sensitivity to Cd(II) and Hg(II) ions for a fluorescent probe. This new kind of nanocrystal probe is hardly interfered by a range of commonly encountered metal ions. Its advantages were demonstrated by determining Cd(II) and Hg(II) ions in spiked serum samples.

Dually emitting nanoclusters composed of gold-nickel alloys are shown to act as very sensitive fluorescent probes for the detection of Cd(II) and Hg(II) ions.






Similar content being viewed by others
References
Kim HN, Ren WX, Kim JS, Yoon J (2012) Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem Soc Rev 41:3210
Liang Y, Kong B, Zhu A, Wang Z, Tian Y (2012) A facile and efficient strategy for photoelectrochemical detection of cadmium ions based on in situ electrodeposition of CdSe clusters on TiO2 nanotubes. Chem Commun 48:245
Guo Y, Zhang Y, Shao H, Wang Z, Wang X, Jiang X (2014) Label-free colorimetric detection of cadmium ions in rice samples using gold nanoparticles. Anal Chem 86:8530
Wang ZX, Ding SN (2014) One-pot green synthesis of high quantum yield oxygen-doped, nitrogen-rich, photoluminescent polymer carbon nanoribbons as an effective fluorescent sensing platform for sensitive and selective detection of silver(I) and mercury(II) ions. Anal Chem 86:7436
Wang H, Kang B, Chancellor TF, Lele TP, Tseng Y, Ren F (2007) Fast electrical detection of Hg(II) ions with AlGaN∕GaN high electron mobility transistors. Appl Phys Lett 91:042114
Anthemidis AN, Karapatouchas CP (2008) Flow injection on-line hydrophobic sorbent extraction for flame atomic absorption spectrometric determination of cadmium in water samples. Microchim Acta 160:455
Shamsipur M, Rajabi HR, Beyzavi MH, Sharghi H (2013) Bulk polymer nanoparticles containing a tetrakis (3-hydroxyphenyl)porphyrin for fast and highly selective separation of mercury ions. Microchim Acta 180:791
Katarina RK, Takayanagi T, Oshima M, Motomizu S (2006) Synthesis of a chitosan-based chelating resin and its application to the selective concentration and ultratrace determination of silver in environmental water samples. Anal Chim Acta 558:246
Davis AC, Calloway CP, Jones BT (2007) Direct determination of cadmium in urine by tungsten-coil inductivily coupled plasma atomic emission spectrometry using palladium as a permanent modifier. Talanta 71:1144
Guo S, Wang E (2011) Noble metal nanomaterials: controllable synthesis and application in fuel cells and analytical sensors. Nano Today 6:240
Xu H, Suslick KS (2010) Water-soluble fluorescent silver nanoclusters. Adv Mater 22:1078
Chen WY, Lan GY, Chang HT (2011) Use of fluorescent DNA-templated gold/silver nanoclusters for the detection of sulfide ions. Anal Chem 83:9450
Wang ZX, Zheng CL, Ding SN (2014) Label-free detection of sulfide ions based on fluorescence quenching of unmodified core-shell Au@Ag nanoclusters. RSC Adv 4:9825
Le Guével X, Hötzer B, Jung G, Hollemeyer K, Trouillet V, Schneider M (2011) Formation of fluorescent metal (Au, Ag) nanoclusters capped in bovine serum albumin followed by fluorescence and spectroscopy. J Phys Chem C 115:10955
Chen YN, Chen PC, Wang CW, Lin YS, Ou CM, Ho LC, Chang HT (2014) One-pot synthesis of fluorescent BSA-Ce/Au nanoclusters as ratiometric pH probes. Chem Commun 50:8571
Zhang N, Si Y, Sun Z, Chen L, Li R, Qiao Y, Wang H (2014) Rapid, selective, and ultrasensitive fluorimetric analysis of mercury and copper levels in blood using bimetallic gold-silver nanoclusters with “silver effect”-enhanced red fluorescence. Anal Chem 86:11714
Varriale A, Staiano M, Rossi M, D’Auria S (2007) High-affinity binding of cadmium ions by mouse metallothionein prompting the design of a reversed-displacement protein-based fluorescence biosensor for cadmium detection. Anal Chem 79:5760
Xi J, Zheng Y, Ying JY (2010) Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+-Au+ interactions. Chem Commun 46:961
Xu YL, Sherwood J, Qin Y, Crowley D, Bonizzonic M, Bao YP (2014) The role of protein characteristics in the formationand fluorescence of Au nanoclusters. Nanoscale 6:1515
Wei H, Wang ZD, Yang LM, Tian SL, Hou CJ, Lu Y (2010) Lysozyme-stabilized gold fluorescent cluster: synthesis and application as Hg2+ sensor. Analyst 135:1406
Sun CJ, Yang H, Yuan Y, Tian X, Wang LM, Guo Y, Xu L, Lei JL, Gao N, Anderson GJ, Liang XJ, Chen CY, Zhao YL, Nie GJ (2011) Controlling assembly of paired gold clusters within apoferritin nanoreactor for in vivo kidney targeting and biomedical imaging. J Am Chem Soc 133:8617
Xie J, Zheng Y, Ying JY (2009) Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc 131:888
Duff DG, Baiker A, Edwards PP (1993) A new hydrosol of gold clusters 1. formation and particle-size variation. Langmuir 9:2301
Zhou L, Lin Y, Huang Z, Ren J, Qu X (2012) Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun 48:1147
Hu X, Zhu K, Guo Q, Liu Y, Ye M, Sun Q (2014) Ligand displacement-induced fluorescence switch of quantum dots for ultrasensitive detection of cadmium ions. Anal Chim Acta 812:191
Browning C, Hudson JM, Reinheimer EW, Kuo FL, McDougald RN, Rabaâ H, Pan H, Bacsa J, Wang X, Dunbar KR, Shepherd ND, Omary MA (2014) Synthesis, spectroscopic properties, and photoconductivity of black absorbers consisting of Pt(bipyridine)(dithiolate) charge transfer complexes in the presence and absence of nitrofluorenone acceptors. J Am Chem Soc 136:16185
Saleh SM, Ali R, Wolfbeis OS (2011) Quenching of the luminescence of upconverting luminescent nanoparticles by heavy metal ions. Chem Eur J 17:14611
Cano-Raya C, Fernandez Ramos MD, Capitan Vallvey LF, Wolfbeis OS, Schaeferling M (2005) Fluorescence quenching of the europium tetracycline hydrogen peroxide complex by copper(II) and other metal ions. Appl Spectrosc 59:1209
Huber C, Werner T, Krause C, Wolfbeis OS (2003) Serum chloride optical sensors based on dynamic quenching of the fluorescence of photo-immobilized lucigenin. Microchim Acta 142:245
Wang X, Stolwijk JA, Lang T, Sperber M, Meier RJ, Wegener J, Wolfbeis OS (2012) Ultra-small, highly stable and sensitive dual nanosensors for imaging intracellular oxygen and pH in cytosol. J Am Chem Soc 134:17011
Banerjee S, Kar S, Santra S (2008) A simple strategy for quantum dot assisted selective detection of cadmium ions. Chem Commun 26:3037
Bronson RT, Michaelis DJ, Lamb RD, Husseini GA, Farnsworth PB, Linford MR, Izatt RM, Bradshaw JS, Savage PB (2005) Efficient immobilization of a cadmium chemosensor in a thin film: generation of a cadmium sensor prototype. Org Lett 7:1105
Huston ME, Engleman C, Czarnik AW (1990) Chelatoselective fluorescence perturbation in anthrylazamacrocycle conjugate probes: electrophilic aromatic cadmiation. J Am Chem Soc 112:7054
Wang B, Wasielewski MR (1997) Design and synthesis of metal ion-recognition-induced conjugated polymers: an approach to metal ion sensory materials. J Am Chem Soc 119:12
Winkler JD, Bowen CM, Michelet V (1998) Photodynamic fluorescent metal ion sensors with parts per billion sensitivity. J Am Chem Soc 120:3237
Pyykkö P (2004) Theoretical chemistry of gold. Angew Chem Int Ed 43:4412
Kirner SV, Guldi DM, Megiatto JJD, Schuster DI (2015) Synthesis and photophysical properties of new catenated electron donor-acceptor materials with magnesium and free base porphyrins as donors and C60 as the acceptor. Nanoscale 7:1145
Gust D, Moore TA, Moore AL (2009) Solar fuels via artificial photosynthesis. Acc Chem Res 42:1890
Guldi DM (2002) Fullerene-porphyrin architectures; photosynthetic antenna and reaction center models. Chem Soc Rev 31:22
Zhao HX, Liu LQ, Liu ZD, Wang Y, Zhao XJ, Huang CZ (2011) Highly selective detection of phosphate in very complicated matrixes with an off-on fluorescent probe of europium-adjusted carbon dots. Chem Commun 47:2604
Wang X, Cao L, Lu FS, Meziani MJ, Li HT, Qi G, Zhou B, Harruff BA, Kermarrec F, Sun YP (2009) Photoinduced electron transfers with carbon dots. Chem Commun 25:3774
Acknowledgments
This work was supported by the National High Technology Research and Development Program (“863” Program) of China (2015AA020502), the Fundamental Research Funds for the Southeast Universities (CXLX13-087), the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University, and the Open Research Fund of Key Laboratory of Energy Thermal Conversion and Control of Ministry of Education, Southeast University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhong-Xia Wang and Shou-Nian Ding contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 2063 kb)
Rights and permissions
About this article
Cite this article
Wang, ZX., Guo, YX. & Ding, SN. Fluorometric determination of cadmium(II) and mercury(II) using nanoclusters consisting of a gold-nickel alloy. Microchim Acta 182, 2223–2231 (2015). https://doi.org/10.1007/s00604-015-1563-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1563-z