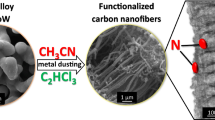
Abstract
Arrays made from quasi-aligned nanofibers consisting of a TiC/C composite were produced directly on a titanium alloy substrate by a thermochemical process. Their morphology, structure and composition were characterized by electron microscopy, X-ray diffraction and X-ray photoelectron spectroscopy. The arrays were directly utilized as an electrode without further treatment and display high catalytic activity in terms of hydrazine oxidation. The low overpotential decreases gradually when increasing pH values from 5 to 10. The detection range is linear from 0.1 to 1,635 μM concentrations, and the detection limit is as low as 0.026 μM (S/N = 3). The selectivity of the electrode and its general performance and stability are very good. The improved electrochemical properties of the new electrode are attributed to the synergic effect of the highly conducting TiC nanowire core and an abundant amount of edge-plane-like defects on the carbon shells.

Quasi-alinged core-shell TiC/C nanofiber arrays were produced by a simple thermochemical method. The nanofiber arrays display electrocatalytic activity towards oxidation of hydrazine with a wide linear range and a very low detection limit of 0.026 μM (S/N = 3).





Similar content being viewed by others
References
Yamada K, Yasuda K, Fujiwara N, Siroma Z, Tanaka H, Miyazaki Y, Kobayashi T (2003) Potential application of anion-exchange membrane for hydrazine fuel cell electrolyte. Electrochem Commun 5:892–896
Yin WX, Li ZP, Zhu JK, Qin HY (2008) Effects of NaOH addition on performance of the direct hydrazine fuel cell. J Power Sourc 182:520–523
Maleki N, Safavi A, Farjami E, Tajabadi F (2008) Palladium nanoparticle decorated carbon ionic liquid electrode for highly efficient electrocatalytic oxidation and determination of hydrazine. Anal Chim Acta 611:151–155
Garrod S, Ballard ME, Nicholls AW, Connor SC, Connelly J, Nicholson JK, Holmes E (2004) Integrated metabonomic analysis of the multiorgan effects of hydrazine toxicity in the rat. Chem Res Toxicol:115–122
Batchelor-McAuley C, Banks CE, Simm AO, Jones TGJ, Compton RG (2006) The electroanalytical detection of hydrazine: a comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated BDD microdisc array. Analyst 131:106–110
Umar A, Rahman MM, Kim SH, Hahn YB (2008) Zinc oxide nanonail based chemical sensor for hydrazine detection. Chem Commun:166–168
Zheng XW, Zhang ZJ, Guo ZH, Wang Q (2002) Flow-injection electrogenerated chemiluminescence detection of hydrazine based on its in-situ electrochemical modification at a pre-anodized platinum electrode. Analyst 127:1375–1379
Mori M, Tanaka K, Xu Q, Ikedo M, Taoda H, Hu W (2004) Highly sensitive determination of hydrazine ion by ion-exclusion chromatography with ion-exchange enhancement of conductivity detection. J Chromatogr A 1039:135–139
Ensafi AA, Rezaei B (1998) Flow injection determination of hydrazine with fluorimetric detection. Talanta 47:645–649
Chen XT, Xiang Y, Li ZF, Tong AJ (2008) Sensitive and selective fluorescence determination of trace hydrazine in aqueous solution utilizing 5-chlorosalicylaldehyde. Anal Chim Acta 625:41–46
Ni YH, Zhu JS, Zhang L, Hong JM (2010) Hierarchical ZnO micro/nanoarchitectures: hydrothermal preparation, characterization and application in the detection of hydrazine. CrystEngComm 12:2213–2218
Quintino MSM, Araki K, Toma HE, Angnes L (2008) New hydrazine sensors based on electropolymerized meso-tetra (4-sulphonatephenyl)porphyrinate manganese (III)/silver nanomaterial. Talanta 74:730–735
Raoof JB, Ojani R, Ramine M (2007) Electrocatalytic oxidation and voltammetric determination of hydrazine on the tetrabromo-p-benzoquinone modified carbon paste electrode. Electroanalysis 19:597–603
Fang B, Zhang CH, Zhang W, Wang GF (2009) A novel hydrazine electrochemical sensor based on a carbon nanotube-wired ZnO nanoflower-modified electrode. Electrochim Acta 55:178–182
Jena BK, Raj CR (2007) Ultrasensitive nanostructured platform for the electrochemical sensing of hydrazine. J Phys Chem C 111:6228–6232
Wang Y, Wan Y, Zhang D (2010) Reduced graphene sheets modified glassy carbon electrode for electrocatalytic oxidation of hydrazine in alkaline media. Electrochem Commun 12:187–190
Wang GF, Gu AX, Wang W, Wei Y, Wu JJ, Wang GZ, Zhang XJ, Fang B (2009) Copper oxide nanoarray based on the substrate of Cu applied for the chemical sensor of hydrazine detection. Electrochem Commun 11:631–634
Prabakar SJR, Narayanan SS (2008) Amperometric determination of hydrazine using a surface modified nickel hexacyanoferrate graphite electrode fabricated following a new approach. J Electroanal Chem 617:111–120
Ardakani MM, Karami PE, Rahimi P, Zare HR, Naeimi H (2007) Electrocatalytic hydrazine oxidation on quinizarine modified glassy carbon electrode. Electrochim Acta 52:6118–6124
Majidi MR, Jouyban A, Asadpour-Zeynali K (2007) Electrocatalytic oxidation of hydrazine at overoxidized polypyrrole film modified glassy carbon electrode. Electrochim Acta 52:6248–6253
Shang NG, Papakonstantinou P, McMullan M, Chu M, Stamboulis A, Potenza A, Dhesi SS, Marchetto H (2008) Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv Funct Mater 18:3506–3514
Salimi A, Miranzadeh L, Hallaj R (2008) Amperometric and voltammetric detection of hydrazine using glassy carbon electrodes modified with carbon nanotubes and catechol derivatives. Talanta 75:147–156
Umar A, Rahman MM, Hahn YB (2009) ZnO nanorods based hydrazine sensors. J Nanosci Nanotechnol 9:4686–4691
Umar A, Rahman MM, Hahn YB (2009) Ultra-sensitive hydrazine chemical sensor based on high-aspect-ratio ZnO nanowires. Talanta 77:1376–1380
Fang XS, Hu LF, Ye CH, Zhang LD (2010) One-dimensional inorganic semiconductor nanostructures: a new carrier for nanosensors. Pure Appl Chem 82:2185–2198
Fang XS, Zhai TY, Gautam UK, Li L, Wu LM, Bando Y, Golberg D (2011) ZnS nanostructures: from synthesis to applications. Prog Mater Sci 56:175–287
Liu JP, Li YY, Jiang JA, Huang XT (2010) C@ZnO nanorod array-based hydrazine electrochemical sensor with improved sensitivity and stability. Dalton Trans 39:8693–8697
Hu LS, Huo KF, Chen RS, Zhang XM, Fu JJ, Chu PK (2010) Core-shell TiC/C quasi-aligned nanofiber arrays on biomedical Ti6Al4V for sensitive electrochemical biosensing. Chem Commun 46:6828–6830
Zhang CH, Wang GF, Ji YL, Liu M, Feng YH, Zhang ZD, Fang B (2010) Enhancement in analytical hydrazine based on gold nanoparticles deposited on ZnO-MWCNTs films. Sensor Actuator B-Chem 50:247–253
Wang MF, Wang C, Wang GF, Zhang W, Bin F (2010) Synthesis of MnO2/MWNTs nanocomposites using a sonochemical method and application for hydrazine detection. Electroanalysis 22:1123–1129
Braude EA, Nachod FC (1955) Determination of organic structures by physical methods/editors. Academic, New York
Bard AJ (1963) Chronopotentiometric oxidation of hydrazine at a platinum electrode. Anal Chem 35:1602–1607
Yi QF, Yu WQ (2009) Nanoporous gold particles modified titanium electrode for hydrazine oxidation. J Electroanal Chem 633:159–164
Umasankar Y, Huang TY, Chen SM (2011) Vitamin B-12 incorporated with multiwalled carbon nanotube composite film for the determination of hydrazine. Anal Biochem 408:297–303
Wang C, Zhang L, Guo ZH, Xu JG, Wang HY, Zhai KF, Zhuo X (2010) A novel hydrazine electrochemical sensor based on the high specific surface area graphene. Microchim Acta 169:1–6
Acknowledgments
This work was jointly financially supported by National Natural Science Foundation of China (50902104), City University of Hong Kong Strategic Research Grant (SRG) No. 7008009, National High Technology Research and Development Program of China No.2009AA02Z416, and Hubei Province Natural Science Foundation (No. 2010CDB03402).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, W., Huo, K., Jiang, Y. et al. Arrays of nanofibers composed of a TiC core and a carbon coating for sensitive electrochemical detection of hydrazine. Microchim Acta 175, 137–143 (2011). https://doi.org/10.1007/s00604-011-0664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-011-0664-6