Abstract
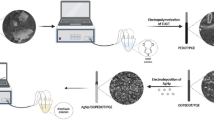
Au-Pd bimetallic nanoparticles were prepared in the presence of an amine-terminated dendrimer of a fourth generation poly(amidoamine) type. A biosensor was fabricated by immobilizing dsDNA on a thin layer of a dendrimer-encapsulated bimetallic nanoparticles (Au-Pd) in a chitosan composite on a glassy carbon electrode. The biosensor was evaluated by square wave voltammetry for the determination of the oxidative damage of immobilized DNA and the antioxidant capacity of sericin. The biosensor is shown to be suitable for the rapid detection of DNA damage and the assessment of the antioxidant capacity of sericin.








Similar content being viewed by others
References
Mello LD, Kubota LT (2007) Biosensors as a tool for the antioxidant status evaluation. Talanta 72:335
Ames BN (1983) Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science 221:1256
Niki E, Shimaski H, Mino M (1994) Antioxidantism-free radical and biological defence. Gakkai Syuppn Center, Tokyo: 3
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables-the millennium’s health. Int J Food Sci Tech 36:703
Yang CS (1997) Inhibition of carcinogenesis by tea. Nature 389:134
Sun B, Fukuhara M (1997) Effects of co-administration of butylated hydroxytoluene, butylated hydroxyanisole and flavonoids on the activation of mutagens and drug-metabolizing enzymes in mice. Toxicology 122:61
Hirose M, Takesada Y, Tanaka H, Tamano S, Kato T, Shirai T (1998) Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multi-organ carcinogenesis model. Carcinogenesis 19:207
Mello LD, Sotomayor M, Kubota LT (2003) HRP-based amperometric biosensor for the polyphenols determination in vegetables extract. Sens Actuator B 96:636
Campanella L, Martini E, Tomassetti M (2005) Antioxidant capacity of the algae using a biosensor method. Talanta 66:902
Wu JH, Wang Z, Xu SY (2007) Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem 103:1255
Kato N, Sato S, Yamanaka A, Yamada H, Fuwa N, Nomura M (1998) Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci Biotechnol Biochem 62:145
Zhaorigetu S, Sasaki M, Watanabe H, Kato N (2001) Supplemental silk protein, sericin, suppresses colon tumorigenesis in 1, 2-dimethylhydrazine-treated mice by reducing oxidative stress and cell proliferation. Biosci Biotechnol Biochem 65:2181
Sasaki M, Yamada H, Kato N (2000) Consumption of silk protein, sericin elevates intestinal absorption of zinc, iron, magnesium and calcium in rats. Nutr Res 20:1505
Zhaorigetu S, Sasaki M, Kato N (2007) Consumption of sericin suppresses colon oxidative stress and aberrant crypt foci in 1, 2-dimethylhydrazine-treated rats by colon undigested sericin. J Nutr Sci Vitaminol 53:297
Sarovart S, Sudatis B, Meesilpa P, Grady BP, Magaraphan R (2003) The use of sericin as an antioxidant and antimicrobial for polluted air treatment. Rev Adv Mater Sci 5:193
Cadet J, Douki T, Gasparutto D, Ravanat JL (2003) Oxidative damage to DNA: formation, measurement and biochemical features. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis 531:5
Korbut O, Bučková M, Tarapčik P, Labuda J J, Gründler P (2001) Damage to DNA indicated by an electrically heated DNA-modified carbon paste electrode. J Electroanal Chem 506:143
Collins A (2000) Comparison of different methods of measuring 8-oxoguanine as a marker of oxidative DNA damage. Free Radical Res 32:333
Ján L, Renáta O, Júlia G (2009) DNA-based biosensor for the detection of strong damage to DNA by the quinazoline derivative as a potential anticancer agent. Microchim Acta 164:371
Zheng Y, Yang C, Pu W, Zhang J (2009) Charbon nanotube-based DNA biosensor for monitoring phenolic pollutants. Microchim Acta 166:21
Mello LD, Hernandez S, Marrazza G, Mascini M, Kubota LT (2006) Investigations of the antioxidant properties of plant extracts using a DNA-electrochemical biosensor. Biosens Bioelectron 21:1374
Liu J, Su B, Lagger G, Tacchini P, Girault HH (2006) Antioxidant redox sensors based on DNA modified carbon screen-printed electrodes. Anal Chem 78:6879
Rusling JF (2004) Sensors for toxicity of chemicals and oxidative stress based on electrochemical catalytic DNA oxidation. Biosens Bioelectron 20:1022
Yang J, Yang T, Feng Y, Jiao K (2007) A DNA electrochemical sensor based on nanogold-modified poly-2, 6-pyridinedicarboxylic acid film and detection of PAT gene fragment. Anal Biochem 365:24
Yoon HC, Kim HS (2000) Multilayered assembly of dendrimers with enzymes on gold: thickness-controlled biosensing interface. Anal Chem 72:922
Zhu N, Gu Y, Chang Z, He P, Fang Y (2006) PAMAM dendrimers-based DNA biosensors for electrochemical detection of DNA hybridization. Electroanalysis 18:2107
Li A, Yang F, Ma Y, Yang X (2007) Electrochemical impedance detection of DNA hybridization based on dendrimer modified electrode. Biosens Bioelectron 22:1716
Shi W, Ai S, Li J, Zhu L (2008) Electrochemical biosensor based on dendrimer Immobilized deoxyribonucleic acid. Chinese J Anal Chem 36:335
Li G, Li X, Wan J, Zhang S (2009) Dendrimers-based DNA biosensors for highly sensitive electrochemical detection of DNA hybridization using reporter probe DNA modified with Au nanoparticles. Biosens Bioelectron 24:3281
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648
Qian L, Yang X (2008) Dendrimer films as matrices for electrochemical fabrication of novel gold/palladium bimetallic nanostructures. Talanta 74:1649
Tomalia DA, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1986) Dendritic macromolecules: synthesis of starburst dendrimers. Macromolecules 19:2466
Esumi K, Suzuki A, Yamahira A, Torigoe K (2000) Role of poly (amidoamine) dendrimers for preparing nanoparticles of gold, platinum, and silver. Langmuir 16:2604
Kim YG, Oh SK, Crooks RM (2004) Preparation and characterization of 1 2 nm dendrimer-encapsulated gold nanoparticles having very narrow size distributions. Chem Mater 16:167
Scott RWJ, Ye H, Henriquez RR, Crooks RM (2003) Synthesis, characterization, and stability of dendrimer-encapsulated palladium nanoparticles. Chem Mater 15:3873
Lebedeva OV, Kim BS, Gröhn F, Vinogradova OI (2007) Dendrimer-encapsulated gold nanoparticles as building blocks for multilayer microshells. Polymer 48:5024
Xu C, Cai H (2001) Immobilization of ssDNA on chitson modified electrode. Fresenius’ J Anal Chem 369:428
Xu Y, Jiang Y, Cai H, He PG, Fang YZ (2004) Electrochemical impedance detection of DNA hybridization based on the formation of M-DNA on polypyrrole/carbon nanotube modified electrode. Anal Chim Acta 516:19
Dharuman V, Grunwald T, Nebling E, Albers J, Blohm L, Hintsche R (2005) Label-free impedance detection of oligonucleotide hybridisation on interdigitated ultramicroelectrodes using electrochemical redox probes. Biosens Bioelectron 21:645
Ghanbari Kh, Bathaie SZ, Mousavi MF (2008) Electrochemically fabricated polypyrrole nanofiber-modified electrode as a new electrochemical DNA biosensor. Biosens Bioelectron 23:1825
Lloyd DR, Phillips DH (1999) Oxidative DNA damage mediated by copper (II), iron (II) and nickel (II) Fenton reactions: evidence for site-specific mechanisms in the formation of double-strand breaks, 8-hydroxydeoxyguanosine and putative intrastrand cross-links. Mutation Research-Fundamental and Molecular Mechanisms of Mutagenesis 424:23
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 20775044), and the Natural Science Foundation of Shandong Province (No. Y2006B20).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, P., Ai, S., Yin, H. et al. Evaluation of DNA damage and antioxidant capacity of sericin by a DNA electrochemical biosensor based on dendrimer-encapsulated Au-Pd/chitosan composite. Microchim Acta 168, 347–354 (2010). https://doi.org/10.1007/s00604-009-0280-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-009-0280-x