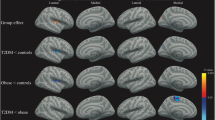
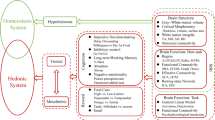
Abstract
Obesity and type 2 diabetes are associated with greater risk of brain damage. Over the last decade, functional imaging techniques (functional magnetic resonance imaging, fMRI, positron emission tomography, PET, electroencephalography, magnetoencephalography, near infrared spectroscopy) have been exploited to better characterize behavioral and cognitive processes, by addressing cerebral reactions to a variety of stimuli or tasks, including hormones and substrates (e.g., glucose, insulin, gut peptides), environmental cues (e.g., presentation of sensory stimuli), and cognitive tasks. Among these techniques, fMRI and PET are most commonly used, and this review focuses on results obtained with these techniques in relation to brain substrate metabolism, appetite control and food intake, and cognitive decline in obesity and type 2 diabetes. The available knowledge indicates that there are a series of cerebral abnormalities associating with, or preceding obesity and type 2 diabetes, including impaired substrate handling, insulin resistance, disruption of inter-organ cross-talk and of resting state networking. Some of these abnormalities are reversed by metabolic interventions, suggesting that they are partly a consequence rather than cause of disease. Therefore, causal implications and mechanisms remain to be determined.


Similar content being viewed by others
References
Amaro E Jr, Barker GJ (2006) Study design in fMRI: basic principles. Brain Cogn 60(3):220–232. https://doi.org/10.1016/j.bandc.2005.11.009
Iozzo P, Guiducci L, Guzzardi MA, Pagotto U (2012) Brain PET imaging in obesity and food addiction: current evidence and hypothesis. Obes Facts 5(2):155–164. https://doi.org/10.1159/000338328
Hirvonen J, Virtanen KA, Nummenmaa L et al (2011) Effects of insulin on brain glucose metabolism in impaired glucose tolerance. Diabetes 60(2):443–447. https://doi.org/10.2337/db10-0940
Liistro T, Guiducci L, Burchielli S et al (2010) Brain glucose overexposure and lack of acute metabolic flexibility in obesity and type 2 diabetes: a PET-[18F]FDG study in Zucker and ZDF rats. J Cereb Blood Flow Metab 30(5):895–899. https://doi.org/10.1038/jcbfm.2010.27
Ferrannini E, Bjorkman O, Reichard GA Jr et al (1985) The disposal of an oral glucose load in healthy subjects. A quantitative study. Diabetes 34(6):580–588
Eastman RC, Carson RE, Gordon MR et al (1990) Brain glucose metabolism in noninsulin-dependent diabetes mellitus: a study in Pima Indians using positron emission tomography during hyperinsulinemia with euglycemic glucose clamp. J Clin Endocrinol Metab 71(6):1602–1610. https://doi.org/10.1210/jcem-71-6-1602
Tuulari JJ, Karlsson HK, Hirvonen J et al (2013) Weight loss after bariatric surgery reverses insulin-induced increases in brain glucose metabolism of the morbidly obese. Diabetes 62(8):2747–2751. https://doi.org/10.2337/db12-1460
Rebelos E, Bucci M, Immonen H et al (2017) Endogenous glucose production is independently associated to brain glucose uptake in morbidly obese subjects. Diabetologia 60:(S80–81)
Bingham EM, Hopkins D, Smith D et al (2002) The role of insulin in human brain glucose metabolism: an 18fluoro-deoxyglucose positron emission tomography study. Diabetes 51(12):3384–3390
Honkala SM, Johansson J, Motiani KK et al (2017) Short-term interval training alters brain glucose metabolism in subjects with insulin resistance. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X17734998
Guzzardi MA, Sanguinetti E, Bartoli A et al (2017) Elevated glycemia and brain glucose utilization predict BDNF lowering since early life. J Cereb Blood Flow Metab. https://doi.org/10.1177/0271678X17697338
Sanguinetti E, Liistro T, Mainardi M et al (2016) Maternal high-fat feeding leads to alterations of brain glucose metabolism in the offspring: positron emission tomography study in a porcine model. Diabetologia 59(4):813–821. https://doi.org/10.1007/s00125-015-3848-5
Heni M, Wagner R, Kullmann S et al (2017) Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66(7):1797–1806. https://doi.org/10.2337/db16-1380
Sanguinetti E, Guzzardi MA, Ditaranto F et al (2017) Maternal high-fat feeding and/or overweight and the early programming of metabolic and cognitive risk in the offspring: a study in mice and human infants. Obes Facts 10(suppl 1):82
Karmi A, Iozzo P, Viljanen A et al (2010) Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59(9):2171–2177. https://doi.org/10.2337/db09-0138
Berthoud HR, Munzberg H, Morrison CD (2017) Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology 152(7):1728–1738. https://doi.org/10.1053/j.gastro.2016.12.050
Matsuda M, Liu Y, Mahankali S et al (1999) Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48(9):1801–1806
Vidarsdottir S, Smeets PA, Eichelsheim DL et al (2007) Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes 56(10):2547–2550. https://doi.org/10.2337/db07-0193
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008) Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117(4):924–935. https://doi.org/10.1037/a0013600
Stoeckel LE, Weller RE, Cook EW III, Twieg DB, Knowlton RC, Cox JE (2008) Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41(2):636–647. https://doi.org/10.1016/j.neuroimage.2008.02.031
Scharmuller W, Ubel S, Ebner F, Schienle A (2012) Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett 518(2):106–110. https://doi.org/10.1016/j.neulet.2012.04.063
Killgore WD, Young AD, Femia LA et al (2003) Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage 19(4):1381–1394
Wang GJ, Volkow ND, Telang F et al (2004) Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 21(4):1790–1797. https://doi.org/10.1016/j.neuroimage.2003.11.026
Fuhrer D, Zysset S, Stumvoll M (2008) Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 16(5):945–950. https://doi.org/10.1038/oby.2008.33
O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ (2002) Neural responses during anticipation of a primary taste reward. Neuron 33(5):815–826
Burger KS, Stice E (2014) Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage 99:122–128. https://doi.org/10.1016/j.neuroimage.2014.05.066
Wang GJ, Volkow ND, Felder C et al (2002) Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 13(9):1151–1155
Volkow ND, Wang GJ, Fowler JS, Telang F (2008) Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363(1507):3191–3200. https://doi.org/10.1098/rstb.2008.0107
DelParigi A, Chen K, Salbe AD, Reiman EM, Tataranni PA (2005) Sensory experience of food and obesity: a positron emission tomography study of the brain regions affected by tasting a liquid meal after a prolonged fast. Neuroimage 24(2):436–443. https://doi.org/10.1016/j.neuroimage.2004.08.035
Wang GJ, Volkow ND, Logan J et al (2001) Brain dopamine and obesity. Lancet 357(9253):354–357
Volkow ND, Wang GJ, Telang F et al (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42(4):1537–1543. https://doi.org/10.1016/j.neuroimage.2008.06.002
Ziauddeen H, Fletcher PC (2013) Is food addiction a valid and useful concept? Obes Rev 14(1):19–28. https://doi.org/10.1111/j.1467-789X.2012.01046.x
Lindgren E, Gray K, Miller G et al (2018) Food addiction: a common neurobiological mechanism with drug abuse. Front Biosci (Landmark Ed) 23:811–836
Berthoud HR (2008) Vagal and hormonal gut–brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20(Suppl 1):64–72. https://doi.org/10.1111/j.1365-2982.2008.01104.x
Goldstone AP, Prechtl CG, Scholtz S et al (2014) Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 99(6):1319–1330. https://doi.org/10.3945/ajcn.113.075291
Malik S, McGlone F, Bedrossian D, Dagher A (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7(5):400–409. https://doi.org/10.1016/j.cmet.2008.03.007
Jones RB, McKie S, Astbury N et al (2012) Functional neuroimaging demonstrates that ghrelin inhibits the central nervous system response to ingested lipid. Gut 61(11):1543–1551. https://doi.org/10.1136/gutjnl-2011-301323
Jastreboff AM, Sinha R, Arora J et al (2016) Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes 65(7):1929–1939. https://doi.org/10.2337/db15-1216
Savage SW, Zald DH, Cowan RL et al (2014) Regulation of novelty seeking by midbrain dopamine D2/D3 signaling and ghrelin is altered in obesity. Obesity (Silver Spring) 22(6):1452–1457. https://doi.org/10.1002/oby.20690
Batterham RL, Ffytche DH, Rosenthal JM et al (2007) PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450(7166):106–109. https://doi.org/10.1038/nature06212
De Silva A, Salem V, Long CJ et al (2011) The gut hormones PYY 3–36 and GLP-1 7–36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab 14(5):700–706. https://doi.org/10.1016/j.cmet.2011.09.010
le Roux CW, Batterham RL, Aylwin SJ et al (2006) Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology 147(1):3–8. https://doi.org/10.1210/en.2005-0972
van Bloemendaal L, RG IJerman, Ten Kulve JS et al (2014) GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 63(12):4186–4196. https://doi.org/10.2337/db14-0849
Farr OM, Sofopoulos M, Tsoukas MA et al (2016) GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia 59(5):954–965. https://doi.org/10.1007/s00125-016-3874-y
Ten Kulve JS, Veltman DJ, van Bloemendaal L et al (2016) Endogenous GLP1 and GLP1 analogue alter CNS responses to palatable food consumption. J Endocrinol 229(1):1–12. https://doi.org/10.1530/JOE-15-0461
Daniele G, Iozzo P, Molina-Carrion M et al (2015) Exenatide regulates cerebral glucose metabolism in brain areas associated with glucose homeostasis and reward system. Diabetes 64(10):3406–3412. https://doi.org/10.2337/db14-1718
Farooqi IS, Bullmore E, Keogh J, Gillard J, O’Rahilly S, Fletcher PC (2007) Leptin regulates striatal regions and human eating behavior. Science 317(5843):1355. https://doi.org/10.1126/science.1144599
Baicy K, London ED, Monterosso J et al (2007) Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci USA 104(46):18276–18279. https://doi.org/10.1073/pnas.0706481104
Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J (2008) Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Investig 118(7):2583–2591. https://doi.org/10.1172/JCI35055
Hinkle W, Cordell M, Leibel R, Rosenbaum M, Hirsch J (2013) Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS One 8(3):e59114. https://doi.org/10.1371/journal.pone.0059114
Frost G, Sleeth ML, Sahuri-Arisoylu M et al (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611. https://doi.org/10.1038/ncomms4611
Byrne CS, Chambers ES, Alhabeeb H et al (2016) Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr 104(1):5–14. https://doi.org/10.3945/ajcn.115.126706
Wang GJ, Zhao J, Tomasi D et al (2018) Effect of combined naltrexone and bupropion therapy on the brain’s functional connectivity. Int J Obes (Lond). https://doi.org/10.1038/s41366-018-0040-2
Scholtz S, Miras AD, Chhina N et al (2014) Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63(6):891–902. https://doi.org/10.1136/gutjnl-2013-305008
van de Sande-Lee S, Pereira FR, Cintra DE et al (2011) Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 60(6):1699–1704. https://doi.org/10.2337/db10-1614
Ochner CN, Kwok Y, Conceicao E et al (2011) Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg 253(3):502–507. https://doi.org/10.1097/SLA.0b013e318203a289
Goldstone AP, Miras AD, Scholtz S et al (2016) Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J Clin Endocrinol Metab 101(2):599–609. https://doi.org/10.1210/jc.2015-2665
Hunt KF, Dunn JT, le Roux CW et al (2016) Differences in regional brain responses to food ingestion after Roux-en-Y gastric bypass and the role of gut peptides: a neuroimaging study. Diabetes Care 39(10):1787–1795. https://doi.org/10.2337/dc15-2721
Musen G, Jacobson AM, Bolo NR et al (2012) Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61(9):2375–2379. https://doi.org/10.2337/db11-1669
Cheke LG, Bonnici HM, Clayton NS, Simons JS (2017) Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia 96:137–149. https://doi.org/10.1016/j.neuropsychologia.2017.01.013
Marder TJ, Flores VL, Bolo NR et al (2014) Task-induced brain activity patterns in type 2 diabetes: a potential biomarker for cognitive decline. Diabetes 63(9):3112–3119. https://doi.org/10.2337/db13-1783
Manschot SM, Brands AM, van der Grond J et al (2006) Brain magnetic resonance imaging correlates of impaired cognition in patients with type 2 diabetes. Diabetes 55(4):1106–1113
Tiehuis AM, van der Graaf Y, Visseren FL et al (2008) Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 39(5):1600–1603. https://doi.org/10.1161/STROKEAHA.107.506089
Debette S, Seshadri S, Beiser A et al (2011) Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 77(5):461–468. https://doi.org/10.1212/WNL.0b013e318227b227
Moran C, Phan TG, Chen J et al (2013) Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36(12):4036–4042. https://doi.org/10.2337/dc13-0143
Willette AA, Xu G, Johnson SC et al (2013) Insulin resistance, brain atrophy, and cognitive performance in late middle-aged adults. Diabetes Care 36(2):443–449. https://doi.org/10.2337/dc12-0922
Bauer CC, Moreno B, Gonzalez-Santos L, Concha L, Barquera S, Barrios FA (2015) Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: a magnetic resonance imaging study in Mexican children. Pediatr Obes 10(3):196–204. https://doi.org/10.1111/ijpo.241
Yau PL, Kang EH, Javier DC, Convit A (2014) Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 22(8):1865–1871. https://doi.org/10.1002/oby.20801
Thompson PM, Hayashi KM, de Zubicaray G et al (2003) Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci 23(3):994–1005
Murray AM, Hsu FC, Williamson JD et al (2017) ACCORDION MIND: results of the observational extension of the ACCORD MIND randomised trial. Diabetologia 60(1):69–80. https://doi.org/10.1007/s00125-016-4118-x
Dickerson BC, Sperling RA (2008) Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: insights from functional MRI studies. Neuropsychologia 46(6):1624–1635. https://doi.org/10.1016/j.neuropsychologia.2007.11.030
Zhou H, Lu W, Shi Y et al (2010) Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci Lett 473(1):5–10. https://doi.org/10.1016/j.neulet.2009.12.057
Cui Y, Jiao Y, Chen YC et al (2014) Altered spontaneous brain activity in type 2 diabetes: a resting-state functional MRI study. Diabetes 63(2):749–760. https://doi.org/10.2337/db13-0519
Xia W, Wang S, Sun Z et al (2013) Altered baseline brain activity in type 2 diabetes: a resting-state fMRI study. Psychoneuroendocrinology 38(11):2493–2501. https://doi.org/10.1016/j.psyneuen.2013.05.012
Cui Y, Li SF, Gu H et al (2016) Disrupted brain connectivity patterns in patients with type 2 diabetes. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4858
Hoth KF, Gonzales MM, Tarumi T, Miles SC, Tanaka H, Haley AP (2011) Functional MR imaging evidence of altered functional activation in metabolic syndrome. AJNR Am J Neuroradiol 32(3):541–547. https://doi.org/10.3174/ajnr.A2315
Kuczynski B, Jagust W, Chui HC, Reed B (2009) An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology 72(8):738–743. https://doi.org/10.1212/01.wnl.0000343005.35498.e5
Willette AA, Bendlin BB, Starks EJ et al (2015) Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol 72(9):1013–1020. https://doi.org/10.1001/jamaneurol.2015.0613
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68(1):51–57. https://doi.org/10.1001/archneurol.2010.225
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Managed by Massimo Federici.
Rights and permissions
About this article
Cite this article
Guzzardi, M.A., Iozzo, P. Brain functional imaging in obese and diabetic patients. Acta Diabetol 56, 135–144 (2019). https://doi.org/10.1007/s00592-018-1185-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-018-1185-0