Abstract
Purpose
Spinal nerve injections have traditionally been performed under fluoroscopic (FL) and computed tomography (CT) guidance. Recently, ultrasound (US)-guided procedures have provided an alternative guidance approach that does not expose the patient and operator to radiation. The aim of this study was to compare the efficacy and safety of US-guided spinal nerve injections compared with FL- or CT-guided spinal nerve injections.
Methods
MEDLINE, Cochrane Library, EMBASE, international clinical trials registry platform (ICTRP) and ClinicalTrials.gov database searches for inclusion until February 2023 were independently performed by two authors using predefined criteria. Randomized controlled trials (RCTs) were included. Primary outcomes were change in pain score (numeric rating scale or visual analogue scale) and major adverse events. Secondary outcomes were procedure time, change in functional disability score and minor adverse events. Meta-analysis was performed using random-effect model. We evaluated the certainty of evidence based on the Grading of Recommendations, Assessment and Development (GRADE) approach.
Results
Eight RCTs involving 962 patients were included. There might be little to no difference in the mean score of the pain change between the US-guided methods and the FL- or CT-guided injections (standard mean difference -0.06; 95% confidence interval [CI] −0.26 to 0.15). US guidance probably reduced major adverse events (0.7% [3/433] and 6.5% [28/433], respectively), reduced procedure time (mean difference −4.19 min; 95% CI −5.09 to −3.30), and probably reduced minor adverse events (2.1% [9/433] and 4.2% [18/433], respectively) compared with FL or CT guidance. There was probably little to no difference in the change in functional disability score with either method.
Conclusion
US-guided spinal nerve injections remained effective and reduced adverse events compared with conventional FL- or CT-guided spinal nerve injections. Further RCTs are required to verify our results.
Study registration
Open Science Forum (Available from: https://osf.io/vt92w/).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cervical and lumbosacral radicular pain is caused by spinal nerve root dysfunction [1, 2] and their prevalence is 1–6% and 3–5%, respectively [3, 4]. This disorder significantly negatively impacts on a patient's physical functioning, mental health, and social participation. Spinal nerve injections, including transforaminal epidural injections, are used to treat cervical spine and lumbosacral radiculopathy [5, 6].
Spinal nerve injections have traditionally been performed under fluoroscopy (FL) or computed tomography (CT) guidance [7, 8]. Recently, ultrasound (US)-guided procedures have provided an alternative approach that does not expose patients or operators to radiation. With this approach, the nerve roots and blood vessels are visible, thus reducing complications [8, 9]. One randomized controlled trial (RCT) reported that US-guided injections significantly reduced the risk of complications and have equivalent accuracy and efficacy compared with CT-guided injections [8]. Another RCT reported that US guidance provided similar pain relief and functional improvements, while facilitating the identification of critical vessels and requiring a shorter procedure time without radiation exposure compared with FL guidance [10]. However, there have been no systematic review and meta-analysis comparing the effectiveness of US guidance with FL or CT guidance for spinal nerve injections. Whether imaging guidance affects the risk of complications during spinal nerve injections remains controversial.
Therefore, this systematic review and meta-analysis aimed to compare the efficacy and safety of US-guided spinal nerve injections with FL- or CT-guided spinal nerve injections.
Methods
This systematic review and meta-analysis of RCTs is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 (PRISMA-2020) (Online Resource 1) [11]. This protocol has been submitted to the Open Science Forum (https://osf.io/vt92w/).
Search method
We searched the following databases: MEDLINE (PubMed); the Cochrane Central Register of Controlled Trials (Cochrane Library), and EMBASE (Dialog); and the World Health Organization International Clinical Trials Platform Search Portal (ICTRP) and ClinicalTrials. gov for ongoing or unpublished trials on 28 February, 2023 (Online Resource 2). We checked each study’s reference list, including international guidelines [12,13,14]. Additionally, we checked each eligible study’s and article citing eligible study’s reference list. The authors of the original studies were asked to provide unpublished or additional data, if necessary.
Inclusion criteria of the articles
We included RCTs that assessed the effectiveness of US guidance versus FL or CT guidance for spinal nerve injections (nerve root and transforaminal epidural injections) in adults. Patients who received interlaminar and caudal epidural blocks were excluded from the study.
The following data were extracted from the trials.
Primary outcomes:
-
1.
Change in pain score: measured using a self-reported scale (e.g. numeric rating scale [NRS] or visual analogue scale [VAS]). To assess changes in the pain scale, we compared the differences in the pain scale before and after the intervention. The pain score at the last follow-up visit was used as the post-intervention score.
-
2.
Major adverse events (e.g., inadvertent vascular puncture, local anesthetic toxicity [central nervous system and cardiovascular symptoms], spinal infarction, visible haematoma, dural puncture, and iatrogenic nerve injury) that require treatment or additional procedures.
Secondary outcomes:
-
1.
Procedure time (minutes): set by the original authors.
-
2.
Change in functional disability score: Neck Disability Index (NDI) [15] was used for cervical spinal nerve block and Oswestry Disability Index (ODI) [16] for lumbar spinal nerve block. To assess changes in functional disability scores, we compared the differences in pre- and post-intervention functional disability scores. The functional disability score at the last follow-up was used as the post-intervention score.
-
3.
Minor adverse events (e.g., transient numbness, vertigo, dizziness, nausea, vomiting, facial flushing, and vasovagal syncope) that did not require any treatment or additional procedures.
Data collection and analysis
The mean difference (MD) and 95% confidence intervals (CIs) were combined for procedure time. The standard mean differences (SMD) and 95% CIs were combined for change in pain score and change in functional disability score. ReviewManager (RevMan 5.4.2, The Cochrane Collaboration, Copenhagen, Denmark) was used to extract data for statistical analyses. A random-effects model was used for all the meta-analyses. For continuous data, missing data were not imputed based on the recommendations of the Cochrane handbook [17]. Chi-squared test was used to test for heterogeneity when the research object, intervention measures, and method of outcome assessment were the same. Statistical heterogeneity was evaluated by visual inspection of the forest plots and calculation of the I2 statistic (0–40%: might not be important; 30–60%: may represent moderate heterogeneity; 50–90%: may represent substantial heterogeneity; 75–100%: considerable heterogeneity) [17]. If there was substantial heterogeneity (I2 > 50%), we assessed its reason.
Two authors (RK and YO) independently screened all titles and abstracts, and assessed the risk of bias using Risk of Bias 2 [18]. Disagreements between the two reviewers were discussed. Two reviewers (RK and NY) evaluated the certainty of evidence based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [19]. Disagreements between the two reviewers were discussed and a third reviewer (JW) acted as an arbiter.
A summary of the findings table was created for the following outcomes based on the Cochrane handbook [17]: change in pain score, major adverse events, procedure time, change in functional disability score, and minor adverse events.
Additional analyses
We performed subgroup analyses of the primary outcomes based on the following factors: cervical or lumbar spinal nerve injections and use of drugs (with steroids vs. without steroids). Additionally, we performed the following sensitivity analyses for the primary outcomes to assess whether the results of the review were robust to the decisions made during the review process: inclusion of studies reporting post intervention values, and exclusion of studies reporting only short-term results (< three months).
Difference between protocol and review
We could not perform the sensitivity analysis for long-term results of change in functional disability score because there were no studies with short term results, and for performers because all studies included senior doctors.
Results
Search results
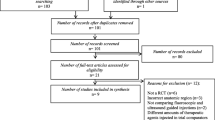
After excluding duplicates, 318 studies were identified in a search conducted in February 2023 (Fig. 1, Online Resource 3). Finally, we included eight reports [8, 10, 20,21,22,23,24,25] in the synthesis. A total of 962 spinal nerve root injections were included: 481 using US and 481 using FL or CT (Table 1). The affected segments were the cervical (n = 5) and lumbar spine (n = 3). The mean follow-up time was 2.4 months (range, 1–6 months). All of the performers of the injections were senior doctors. Of the eight studies, six were performed in an outpatient setting and the remaining two were not reported. The risk of bias for each study is shown in Fig. 2 and Online Resource 4–7.
Primary outcomes
Change in pain score
Eight RCTs (962 participants) reported changes in pain score [8, 10, 20,21,22,23,24,25]. Among them, five studies used the VAS, while three studies used the NRS. The evidence suggests that there was little to no difference in the mean score of the VAS/NRS for the patients’ pain change between the US-guided methods and the FL- or CT-guided injections (SMD − 0.06; 95% CI, -0.26 to 0.15; I2 = 52%; low certainty of evidence) (Fig. 3).
Major adverse events
Six RCTs (866 participants) reported major adverse events [8, 10, 20,21,22, 24]. In the cohort, 0.7% (3/433) had inadvertent vascular puncture with US guidance. Of these, 0.8% (3/393) and 0% (0/40) were cervical and lumbar injections, respectively. In contrast, 6.5% (28/433) experienced major adverse events with FL or CT guidance. Of these, 7.2% (28/393) and 0% (0/40) were cervical and lumbar injections, respectively. With the exception of inadvertent vascular puncture, no major adverse events (e.g., local anesthetic toxicity [central nervous system and cardiovascular symptoms], spinal cord infarction, visible haematoma, dural puncture, or iatrogenic nerve injury) requiring treatment or additional procedures were observed in either group. The US-guided injections probably reduced major adverse events; moderate certainty of evidence (Table 2).
Secondary outcomes
Procedure time
Seven RCTs (865 participants) reported procedure times [8, 10, 21,22,23,24,25]. All trials reported shorter times with US. US-guided injections reduced procedure time compared with the FL- or CT-guided injections (MD − 4.19 min; 95% CI, − 5.09 to − 3.30; I2 = 95%; high certainty of evidence) (Fig. 4a).
Forest plot of included studies comparing ultrasound (US) guided spinal nerve injections with fluoroscopy (FL) or computed tomography (CT) guided spinal nerve injections on the procedure time (a); change in functional disability score (b) (SD. standard deviation; IV, inverse-variance method; CI, confidence interval; df, degree of freedom)
Change in functional disability score
Four RCTs (762 participants) reported change in functional disability score [10, 20, 23, 24]. Among them, three studies of the cervical spine used the NDI, while one study used the ODI. There was probably little to no difference in the mean functional disability score change between the US- and FL- or CT-guided injections (SMD 0.12; 95% CI, − 0.04 to 0.28; I2 = 12%; moderate certainty of evidence) (Fig. 4b).
Minor adverse event
Six RCTs (865 participants) reported minor adverse events.8,10,20–22,24 In the cohort, 2.1% (9/433), all of whom belonged to the cervical group (2.3% [9/393]), experienced drug-induced adverse events such as nausea, vertigo, and flush reaction with US-guided injections. In contrast, 4.2% (18/433), all of whom belonged to the cervical group (4% [18/393]), experienced minor adverse events with FL or CT-guided injections. One case of CT-guided cervical nerve root injection resulted in persistent numbness for one week. The US-guided methods probably reduced minor adverse events; moderate certainty of evidence (Table 2).
Additional analysis
All the pre-specified subgroup analyses for change in pain score, procedure time, and change in functional disability score revealed no significant differences between the subgroups (cervical spine or lumbar spine [p = 0.50, 0.93 and 0.79, respectively]; with or without steroids (p = 0.08 and 0.14, respectively)) (Fig. 5a, b, c; Online Resources 8 and 9).
Forest plot of included studies comparing ultrasound (US) guided spinal nerve injections with fluoroscopy (FL) or computed tomography (CT) guided spinal nerve injections that of subgroup analysis between cervical spine and lumbar spine. Change in pain score (a); Procedure time (b); Change in functional disability score (c) (SD. standard deviation; IV, inverse-variance method; CI, confidence interval; df, degree of freedom)
The post-intervention values for the sensitivity analysis tended to be valid for the FL- or CT-guided injections, although not significantly different in the pain score (p = 0.06), and the FL- or CT-guided injections were significantly more valid for the functional score (p = 0.008) (Online Resources 10 and 11).
Regarding long-term results of the pain score (> three months), the change in pain score was significantly greater with the FL or CT guidance than with US guidance (p = 0.002) (Online Resource 12).
Discussion
The results of this review covering eight RCTs including 962 participants showed that US-guided spinal nerve injections might result in little to no difference in change in pain score compared to the FL- or CT-guidance, and that US-guided injections slightly reduced adverse events and procedure time.
The findings of this review, similar to those of previous studies [8, 10, 20,21,22, 24], suggest that US-guided injections were as effective as FL- or CT-guided injections in reducing pain and improving function. Regarding the pain score, the amount of change in pain score before and after the intervention and the value after the intervention were not significantly different between the US and FL/CT groups. Sensitivity analysis revealed that the long-term results (> three months) of the pain score were more effective in the FL- or CT-guided group. However, the amount of change was small compared to the minimal clinically important differences (MCID) [26, 27]. The post-intervention value in the sensitivity analysis for the functional disability score was significantly smaller in the FL/CT group than in the US group. This may be because the functional disability scores for the FL- or CT-guided groups were lower at the start of the trials; however, there was no significant difference in the amount of change. In the sensitivity analysis, there were statistical differences from the results of the main analysis for the 3-month pain score and the post-intervention functional disability score. However, there was no difference in the amount of change, and the values were small compared to the MCID. Thus, we concluded it to be clinically similar to the results of the main analysis.
Additionally, we observed that the use of US-guided injections probably reduced the incidence of major adverse events more than the use of FL- or CT-guided injections. Similar results were observed for minor adverse events. Major and minor adverse events occurred only in the cervical spine and were not reported in the lumbar spine. Although FL/CT-guided cervical nerve root block is a widely accepted standard procedure, accidental intravascular injections can lead to catastrophic problems such as vertebral artery dissection or spinal cord infarction [28, 29]. In contrast, US-guided injections may avoid injury to vessels, the leading cause of reported complications with spinal nerve injections [8], by facilitating the identification of critical vessels in unexpected locations relative to the foramen or associated nerve. This review showed that US-guided injections are safer than FL- or CT-guided injections, especially in the cervical spine, and might be recommended for future practice. Some clinical guidelines recommend FL- or CT-guidance for spinal nerve injections [12, 14, 30]; however, none have recommended US guidance. In clinical guidelines, US-guidance should be considered and the complications should be discussed along with the efficacy.
Furthermore, we observed that US guidance reduced procedure time. This might be due to the visibility of the nerve roots, blood vessels [10] and needle. The present US technique allows for real-time “in-plane” needle access. This avoids all relevant vessels, as none usually cross the needle path based on normal topography [8]. All performers in this review were senior doctors; thus, it is unclear whether similar results can be achieved with junior doctors, as US guidance is considered a skill-dependent procedure. However, when performed by an experienced doctor under US guidance, the time saved is approximately 5 min, which is clinically useful, especially for outpatient bedside procedures [8]. The elimination of transport time to the fluoroscopy room is another time advantage of the US-guided procedures. The results are applicable to outpatient settings because most study populations in this review were outpatients.
The strength of this study is that, to our knowledge, this is the first systematic review and meta-analysis to evaluate the efficacy and safety of different types of guided spinal nerve injections. We contacted the original authors and searched for potentially unpublished studies whenever possible. Furthermore, this review was based on a rigorous methodology, such as the Cochrane Handbook and GRADE recommendations [17, 19].
However, this review has a couple of limitations. First, many of the overall risk of bias were assessed as concerns because of the non-blinding of the outcome assessor and the selection of the reported results. Therefore, further blinded RCTs with pre-registered protocols are required. Second, difficult cases, such as those with high body mass index or children, were not evaluated. Accordingly, further research is needed to increase the certainty and generalizability of the evidence.
In conclusion, this systematic review and meta-analysis demonstrated that US-guided spinal nerve injections remained effective and reduced adverse events compared with conventional FL- or CT-guided spinal nerve injections. Therefore, physicians can use US-guided spinal nerve injections to reduce adverse events. However, more RCTs blinded to the assessor and pre-registered studies that account for variations in performers and objects are needed to verify the efficacy and safety of spinal nerve root injections.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jensen RK, Kongsted A, Kjaer P, Koes B (2019) Diagnosis and treatment of sciatica. BMJ 367:l6273. https://doi.org/10.1136/bmj.l6273
Woods BI, Hilibrand AS (2015) Cervical radiculopathy: epidemiology, etiology, diagnosis, and treatment. J Spinal Disord Tech 28:E251–E259. https://doi.org/10.1097/BSD.0000000000000284
Mansfield M, Smith T, Spahr N, Thacker M (2020) Cervical spine radiculopathy epidemiology: a systematic review. Musculoskeletal Care 18:555–567. https://doi.org/10.1002/msc.1498
Tarulli AW, Raynor EM (2007) Lumbosacral radiculopathy. Neurol Clin 25:387–405. https://doi.org/10.1016/j.ncl.2007.01.008
Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP (2021) Low back pain. Lancet 398:78–92. https://doi.org/10.1016/S0140-6736(21)00733-9
Yang D, Xu L, Hu Y, Xu W (2022) Diagnosis and treatment of cervical spondylotic radiculopathy using selective nerve root block (SNRB): Where are we now? Pain Ther 11:341–357. https://doi.org/10.1007/s40122-022-00357-1
Rathmell JP, Aprill C, Bogduk N (2004) Cervical transforaminal injection of steroids. Anesthesiology 100:1595–1600. https://doi.org/10.1097/00000542-200406000-00035
Obernauer J, Galiano K, Gruber H, Bale R, Obwegeser AA, Schatzer R, Loizides A (2013) Ultrasound-guided versus computed tomography-controlled periradicular injections in the middle and lower cervical spine: a prospective randomized clinical trial. Eur Spine J 22:2532–2537. https://doi.org/10.1007/s00586-013-2916-0
Korbe S, Udoji EN, Ness TJ, Udoji MA (2015) Ultrasound-guided interventional procedures for chronic pain management. Pain Manag 5:465–482. https://doi.org/10.2217/pmt.15.46
Cui X, Zhang D, Zhao Y, Song Y, He L, Zhang J (2022) An open-label non-inferiority randomized trial comparing the effectiveness and safety of ultrasound-guided selective cervical nerve root block and fluoroscopy-guided cervical transforaminal epidural block for cervical radiculopathy. Ann Med 54:2681–2691. https://doi.org/10.1080/07853890.2022.2124445
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Kreiner DS, Hwang SW, Easa JE, Resnick DK, Baisden JL, Bess S, Cho CH, DePalma MJ, Dougherty P 2nd, Fernand R et al (2014) An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J 14:180–191. https://doi.org/10.1016/j.spinee.2013.08.003
Kjaer P, Kongsted A, Hartvigsen J, Isenberg-Jørgensen A, Schiøttz-Christensen B, Søborg B, Krog C, Møller CM, Halling CMB, Lauridsen HH et al (2017) National clinical guidelines for non-surgical treatment of patients with recent onset neck pain or cervical radiculopathy. Eur Spine J 26:2242–2257. https://doi.org/10.1007/s00586-017-5121-8
Stochkendahl MJ, Kjaer P, Hartvigsen J, Kongsted A, Aaboe J, Andersen M, Andersen MØ, Fournier G, Højgaard B, Jensen MB et al (2018) National Clinical Guidelines for non-surgical treatment of patients with recent onset low back pain or lumbar radiculopathy. Eur Spine J 27:60–75. https://doi.org/10.1007/s00586-017-5099-2
Vernon H, Mior S (1991) The neck disability index: a study of reliability and validity. J Manipulative Physiol Ther 14:409–415
Fairbank JC, Pynsent PB (2000) The Oswestry disability index. Spine (Phila Pa 1976) 25:2940–2952
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors) (2021) Cochrane handbook for systematic reviews of interventions, version 6.2 (updated February 2021). Cochrane. https://training.cochrane.org/handbook/archive/v6.2
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM et al (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. https://doi.org/10.1136/bmj.l4898
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H et al (2011) GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 64:383–394. https://doi.org/10.1016/j.jclinepi.2010.04.026
Jee H, Lee JH, Kim J, Park KD, Lee WY, Park Y (2013) Ultrasound-guided selective nerve root block versus fluoroscopy-guided transforaminal block for the treatment of radicular pain in the lower cervical spine: a randomized, blinded, controlled study. Skeletal Radiol 42:69–78. https://doi.org/10.1007/s00256-012-1434-1
Loizides A, Gruber H, Peer S, Galiano K, Bale R, Obernauer J (2013) Ultrasound guided versus CT-controlled pararadicular injections in the lumbar spine: a prospective randomized clinical trial. AJNR Am J Neuroradiol 34:466–470. https://doi.org/10.3174/ajnr.A3206
Yang G, Liu J, Ma L, Cai Z, Meng C, Qi S, Zhou H et al (2016) Ultrasound-guided versus fluoroscopy-controlled lumbar transforaminal epidural injections: a prospective randomized clinical trial. Clin J Pain 32:103–108. https://doi.org/10.1097/AJP.0000000000000237
Zhang M, Xu Z, Peng Z, Su B, Zhu Y (2022) The efficacy of selective nerve root injection guided by ultrasound or fluoroscopy in patients with chronic low back pain and unilateral radiculopathy. Ann Clin Case Rep 7:2128
Yue L, Zheng S, Hua L, Li H, Yang Y, Li J, He L (2023) Ultrasound-guided versus computed tomography fluoroscopy-assisted cervical transforaminal steroid injection for the treatment of radicular pain in the lower cervical spine: a randomized single-blind controlled noninferiority study. Clin J Pain 39:68–75. https://doi.org/10.1097/AJP.0000000000001091
Plaikner M, Kögl N, Gruber H, Bale R, Ho WM, Skalla-Oberherber E, Loizides A (2023) Ultrasound-guided versus computed tomography-controlled periradicular injections of the first sacral nerve: a prospective randomized clinical trial. Med Ultrason 25:35–41. https://doi.org/10.11152/mu-3827
Pool JJ, Ostelo RW, Hoving JL, Bouter LM, de Vet HC (2008) Minimal clinically important change of the Neck Disability Index and the Numerical Rating Scale for patients with neck pain. Spine (Phila Pa 1976) 32:3047–3051. https://doi.org/10.1097/BRS.0b013e31815cf75b
Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC (2008) Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976) 33:90–94. https://doi.org/10.1097/BRS.0b013e31815e3a10
Wallace MA, Fukui MB, Williams RL, Ku A, Baghai P (2007) Complications of cervical selective nerve root blocks performed with fluoroscopic guidance. AJR Am J Roentgenol 188:1218–1221. https://doi.org/10.2214/AJR.04.1541
Brouwers PJAM, Kottink EJBL, Simon MAM, Prevo RL (2001) A cervical anterior spinal artery syndrome after diagnostic blockade of the right C6-nerve root. Pain 91:397–399. https://doi.org/10.1016/S0304-3959(00)00437-1
Bono CM, Ghiselli G, Gilbert TJ, Kreiner DS, Reitman C, Summers JT, Baisden JL, Easa J, Fernand R, Lamer T et al (2011) An evidence-based clinical guideline for the diagnosis and treatment of cervical radiculopathy from degenerative disorders. Spine J 11:64–72. https://doi.org/10.1016/j.spinee.2010.10.023
Acknowledgements
I would like to thank Alexander Loizides from the Department of Radiology, Medical University of Innsbruck for providing us with the additional data required for this study.
Funding
No funding was received for conducting this study. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Author information
Authors and Affiliations
Contributions
RK: conceptualization, methodology, statistical analysis, writing-original draft, NY: conceptualization, methodology, writing-review & editing, JW: conceptualization, methodology, writing-review & editing, YO: data collection, MH: writing-review & editing, NM: supervision of project.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimura, R., Yamamoto, N., Watanabe, J. et al. Comparative efficacy of ultrasound guidance and fluoroscopy or computed tomography guidance in spinal nerve injections: a systematic review and meta-analysis. Eur Spine J 32, 4101–4110 (2023). https://doi.org/10.1007/s00586-023-07968-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-023-07968-y