Abstract
Purpose
To investigate the impact of early versus delayed surgery on sensory abnormalities in acute traumatic central cord syndrome (ATCCS).
Methods
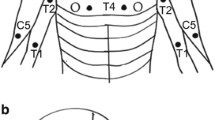
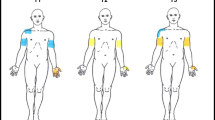
Pressure pain threshold (PPT), temporal summation (TS), conditioned pain modulation (CPM) and pain assessments were performed in 72 ATCCS patients (early vs. delayed surgical treatment: 32 vs. 40) and 72 healthy subjects in this ambispective cohort study. These examinations, along with mechanical detection threshold (MDT) and disabilities of arm, shoulder and hand (DASH), were assessed at 2 years postoperatively.
Results
Preoperatively, more delayed surgical patients had neuropathic pain below level compared with early surgical patients (P < 0.05). Both early and delayed surgical patients showed reduced PPT in common painful areas and increased TS, while reduced CPM only existed in the latter (P < 0.05). Reduced PPT in all tested areas, along with abnormalities in TS and CPM, was observed in patients with durations over 3 months. Both incidences and intensities of pain and pain sensitivities in common painful areas were reduced in both treatment groups postoperatively, but only early surgical treatment improved the CPM and TS. Follow-up analysis demonstrated a higher MDT and lower PPT in hand, greater TS, greater DASH, lower pain intensities and higher incidence of dissatisfaction involving sensory symptoms in delayed surgical patients than in early surgical patients (P < 0.05).
Conclusions
Central hypersensitivity may be involved in the persistence of sensory symptoms in ATCCS, and this augmented central processing may commence in the early stage. Early surgical treatment may reverse dysfunction of endogenous pain modulation, thus reducing the risk of central sensitization and alleviating sensory symptoms.


Similar content being viewed by others
References
Stevens EA, Marsh R, Wilson JA et al (2010) A review of surgical intervention in the setting of traumatic central cord syndrome. Spine J 10:874–880
Ter Wengel PV, Feller RE, Stadhouder A et al (2018) Timing of surgery in traumatic spinal cord injury: a national, multidisciplinary survey. Eur Spine J 27:1831–1838
Anderson KK, Tetreault L, Shamji MF et al (2015) Optimal timing of surgical decompression for acute traumatic central cord syndrome: a systematic review of the literature. Neurosurgery 77:S15–S32
Yelamarthy PKK, Chhabra HS, Vaccaro A et al (2019) Management and prognosis of acute traumatic cervical central cord syndrome: systematic review and spinal cord society-spine trauma study group position statement. Eur Spine J 28:2390–2407
Wilson JR, Witiw CD, Badhiwala J et al (2020) Early surgery for traumatic spinal cord injury: where are we now? Glob Spine J 10:84S-91S
Zheng C, Zhu D, Zhu Y et al (2021) Early surgery improves peripheral motor axonal dysfunction in acute traumatic central cord syndrome: a prospective cohort study. Clin Neurophysiol 132:1398–1406
Chen L, Yang H, Yang T et al (2009) Effectiveness of surgical treatment for traumatic central cord syndrome. J Neurosurg Spine 10:3–8
Siddall PJ, Taylor DA, McClelland JM et al (1999) Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 81:187–197
Hopkins A, Rudge P (1973) Hyperpathia in the central cervical cord syndrome. J Neurol Neurosurg Psychiatry 36:637–642
Merriam WF, Taylor TK, Ruff SJ et al (1986) A reappraisal of acute traumatic central cord syndrome. J Bone Joint Surg Br 68:708–713
Voerman VF, van Egmond J, Crul BJ (2000) Elevated detection thresholds for mechanical stimuli in chronic pain patients: support for a central mechanism. Arch Phys Med Rehabil 81:430–435
Sjölund BH (2002) Pain and rehabilitation after spinal cord injury: the case of sensory spasticity? Brain Res Rev 40:250–256
Liu Y, Shi CG, Wang XW et al (2015) Timing of surgical decompression for traumatic cervical spinal cord injury. Int Orthop 39:2457–2463
Aarabi B, Akhtar-Danesh N, Chryssikos T et al (2020) Efficacy of ultra-early (< 12 h), Early (12–24 h), and late (> 24–138.5 h) surgery with magnetic resonance imaging-confirmed decompression in American spinal injury association impairment scale grades A, B, and C cervical spinal cord injury. J Neurotrauma 37:448–457
Lewis GN, Rice DA, McNair PJ (2012) Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain 13:936–944
Mackey IG, Dixon EA, Johnson K et al (2017) Dynamic quantitative sensory testing to characterize central pain processing. J Vis Exp 120:54452
Ramaswamy S, Wodehouse T (2021) Conditioned pain modulation—a comprehensive review. Neurophysiol Clin 51:197–208
Cathcart S, Winefield AH, Rolan P et al (2009) Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag 14:433–438
Tuveson B, Leffler AS, Hansson P (2000) Influence of heterotopic noxious conditioning stimulation on spontaneous pain and dynamic mechanical allodynia in central post-stroke pain patients. Pain 143:84–91
Siddall PJ, Taylor DA, Cousins MJ (1997) Classification of pain following spinal cord injury. Spinal Cord 35:69–75
Petersen KK, Vaegter HB, Stubhaug A et al (2021) The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain 162:31–44
Irvine KA, Sahbaie P, Ferguson AR et al (2019) Enhanced descending pain facilitation in acute traumatic brain injury. Exp Neurol 320:112976
Irvine KA, Sahbaie P, Ferguson AR et al (2020) Loss of diffuse noxious inhibitory control after traumatic brain injury in rats: a chronic issue. Exp Neurol 333:113428
Tampin B, Briffa NK, Slater H (2013) Self-reported sensory descriptors are associated with quantitative sensory testing parameters in patients with cervical radiculopathy, but not in patients with fibromyalgia. Eur J Pain 17:621–633
Apkarian AV, Stea RA, Bolanowski SJ (1994) Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res 11:259–267
Woolf CJ (2011) Central sensitization: implications for the diagnosis and treatment of pain. Pain 152:S2–S15
Boland RA, Lin CS, Engel S et al (2011) Adaptation of motor function after spinal cord injury: novel insights into spinal shock. Brain 134:495–505
Funding
Financial support from the National Natural Science Foundation of China Science Foundation Project (82072488) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed. The authors alone are responsible for the content and writing of this paper.
Ethical approval
This study protocol was approved by the Ethics Committee of Huashan Hospital (Fudan University, Shanghai, China), and informed consent was obtained from all participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, K., Nie, C., Song, H. et al. Early surgical intervention alleviates sensory symptoms following acute traumatic central cord syndrome. Eur Spine J 32, 608–616 (2023). https://doi.org/10.1007/s00586-022-07447-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-022-07447-w