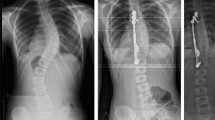
Abstract
Purpose
Growth-friendly spinal implants (GFSI) were established for scoliotic children as an interim solution until definite spinal fusion could be performed during puberty. While deformity control was clearly proven, the effects on vertebral shape and morphology are still unclear. Our prospective study assesses the effect of GFSI with continuous distraction on vertebral body shape and volume in SMA children in comparison with previously untreated age-matched SMA patients.
Methods
Cohort I (n = 19, age 13.2 years) were SMA patients without prior surgical scoliosis treatment. Cohort II (n = 24, age 12.4 years) were children, who had continuous spinal distraction with GFSI for 4.5 years. Radiographic measurements and computed tomography (CT) 3D volume rendering were performed before definite spinal fusion. For cohort II, additional radiographs were analyzed before the first surgical implantation of GFSI, after surgery and every year thereafter.
Results
Our analysis revealed decreased depth and volume in scoliotic patients with prior GFSI compared to scoliotic patients without prior implants. This difference was significant for the lower thoracic and entire lumbar spine. Vertebral body height and pedicle size were unchanged between the two cohorts.
Conclusion
CT data showed volume reduction in the vertebral body in scoliotic children after GFSI treatment. This effect was more severe in the lumbar and lower thoracic area. While vertebral height was identical in both groups, vertebral depth was reduced in the GFSI-treated group. Reduced vertebral depth and altered vertebral morphology should be considered before instrumenting the spine in previously treated scoliotic SMA children.
Level of evidence III
Diagnostic: individual cross-sectional studies with consistently applied reference standard and blinding.
Similar content being viewed by others
Introduction
In their first decade of life, nearly all children with spinal muscular atrophy (SMA) type I or II, a genetic neuromuscular disorder with loss of lower motor neurons and progressive muscle weakness, will develop progressive spinal deformity [1]. This may be life threatening due to lung impairment by severe curves. Because of new treatment options with intrathecal Nusinersen injection [2] or genetic treatment [3], this patient group will most likely survive decades longer than patients before. In 2018, a surgical procedure addressing the problem of progressive spinal deformity by bilateral magnetically controlled devices in combination with rib to pelvis fixation was published [4] after establishing this method in 2011. In SMA children, the advantages of this method are deformity control and reduction in surgical interventions due to noninvasive implant lengthening [5].
Some effects of distraction-based growth-friendly spinal implants (GFSI) on vertebral morphology were described by Hasler et al. [6]. However, the comparison of radiographic findings, such as vertebral height and depth, underlies several factors that make sound conclusion difficult, i.e., focus of X-ray beam, projection artifacts or vertebral body distortion [7, 8].
This study analyzes vertebral shape and volume in age-matched SMA children with and without GFSI for spinal deformity correction. By analyzing a homogeneous cohort of SMA Type II children with spinal deformity, we aimed to reduce additional influencing factors. Therefore, this study design allowed us to examine solely the effect of these implants on the vertebral column.
Material and methods
Cohorts subjected to radiographic analysis and computed tomography
After ethics committee approval, 43 pediatric patients with SMA type I (n = 2) and II (n = 41) were included in a prospective non-randomized cohort study (Table 1). The ethics committee waived the need for informed consent for the study. The participants were informed about the purpose of the study. Cohort I (n = 19) consisted of juvenile SMA patients who initially presented with severe spinal deformity without any prior surgical spine intervention. Late presentation was either due to fear of surgical treatment or because of a refugee status. Cohort II (n = 24) were SMA children, who had received a bilateral GFSI with rib (ribs 2–4) to pelvis fixation at an average age of 7.9 (+ / − 1.9) years. Repeated lengthening procedures with five mm per side were performed every three months. Cobb angles were measured in order to quantify the severity of spinal deformity (Table 1).
In cohort I, ap (anterior–posterior) and lateral radiographs in a standardized sitting position were taken before definitive spinal fusion, as well as computed tomography (CT) (SOMATOM Definition AS, Siemens, Erlangen, Germany) with 0.6 mm slice thickness of the whole spine for pre-surgical evaluation. In cohort II, radiographs were performed before the first surgical implantation of GFSI, after surgery and every year thereafter. To minimize the risk of implant infection [9], GFSI were removed in all children several weeks prior to definitive spinal fusion. Pre-surgical radiologic analysis and CT for pre-surgical evaluation of definitive spinal fusion were performed as in cohort I.
In cohort I, radiographs before definitive spinal fusion were compared with the last radiographic evaluation of cohort II, respectively. Additionally, to assess morphometric changes over time under the influence of GFSI, radiographic measurements of a subgroup of cohort II (n = 18) with a minimum follow-up of four years were compared to data before the first surgery, at approximately two years after surgery and after more than four years. Thirty-two CT scans (16 in each group), which were performed before definitive spinal fusion to plan positioning of screws, were compared for volume data.
Analysis of radiographic measurements and computed tomography
On radiographs, the anterior height and depth at the upper endplate were measured for T1 to L5 in lateral radiographs using Centricity Enterprise Web Version 3.0 (GE Healthcare Medical Systems, Chicago, USA, 2006) and compared between the described groups (Fig. 1). Only heights and depths that could be clearly identified (i.e., vertebrae not twisted) were measured and included in the analysis. (See n-numbers below each column in Fig. 1 and legend of Fig. 2).
Morphometric analysis of vertebral height, depth and volume. Radiographic measurement of vertebral height a revealed no difference between SMA patients with or without prior growth-friendly surgical implants (GFSI) b. In contrast, vertebral depth c was decreased in the patient group with prior GFSI, especially in the lower thoracic and lumbar spine d. Volumetric analysis of CT data e revealed smaller volume of vertebral bodies in the lower thoracic and lumbar spine of patients with prior GFSI f. Vertebral depth and vertebral volume were reduced from T8/T7 downward; p < 0.05 (*), p < 0.01 (**). Whiskers indicate minimum and maximum values. (x/x) represents the n values for each evaluation T1-T12 thoracic vertebra 1–12; L1-L5 lumbar vertebra 1–5
Vertebral height and depth of T4, T12 and L4 in SMA children. In SMA children with growth-friendly spinal implants for deformity correction vertebral height of T4, T12 and L4 increased significantly with a follow-up > 4 years (a–c). In T12 and L4, vertebral height increased significantly within 2-year follow-up. Vertebral depth remained unchanged over the course of > 4 years after GFSI in all vertebrae (d–f). n = 10 (T4), n = 17 (T12), n = 18 (L4) for vertebral height; n = 10 (T4), n = 16 (T12), n = 17 (L4) for vertebral depth. p < 0.01 (**), p < 0.001 (***). Whiskers indicate minimum and maximum values. T4 thoracic vertebra 4; T12 thoracic vertebra 12; L4 lumbar vertebra 4
Software-based semi-automated three-dimensional (3D) reconstruction was performed to compare the volumes of individual vertebrae between the two study groups. CT scans were evaluated using IntelliSpace Portal 9.0 software (Philips Healthcare, Best, the Netherlands) that allows vertebral segmentation and volume measurement [10]. As recommended by the distributor, vertebral bony landmarks were defined for each slice in a 0.6-mm-thick CT scan. The posterior edge of the vertebral body was defined as the posterior border of the volume. Pedicle volume was not included or measured. To optimize measurements, semi-automated recognition from IntelliSpace was used and revised for every coronal, sagittal and transverse reconstruction.
Statistical analysis
The acquired data were statistically analyzed using Excel Version 2010 (Microsoft Cooperation, Redmond, Washington, USA) and GraphPad Prism Version 5 (GraphPad Software Inc. San Diego, California, USA). Because of independent values, for which normal distribution could not be assumed, the Mann–Whitney test was used for statistical comparisons for each individual vertebra in Fig. 1. The Wilcoxon matched-pairs test was used for statistical comparisons in Fig. 2. The test was applied to individually assess growth after 2 years compared to time point 0 and to assess growth after > 4 years compared to time point 0. Box plots in Figs. 1 and 2 indicate median, upper and lower quartile; whiskers indicate minimum and maximum values. Values given in the text are mean ± standard deviation. Statistical significance was defined with levels as p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***).
Results
To assess the effect of GFSI on vertebral dimensions, a total of 43 children with SMA type I or II and spinal deformity were analyzed. Changes in vertebral height and depth were addressed by radiographic analysis; vertebral volume was measured by 3D volume rendering from CT data. All children were non-ambulatory and presented with large C-shaped scoliotic curves.
Radiographic measurements were done in Cohort I and II (Table 1). A subgroup of Cohort II with a minimum follow-up of more than four years was analyzed separately. CT volume data were acquired for 32 SMA children.
Vertebral height did not show any significant differences between both age-matched groups (Cohort I and II) of SMA children (Fig. 1a, b). Vertebral height increased from the thoracic toward the lumbar spine. Radiological measurements on lateral radiographs of vertebral depth showed significantly reduced values in the surgically treated cohort (II) (Fig. 1c,d). This finding was evident below the mid-thoracic area (below T7). Expectantly, vertebral body volume data demonstrated significantly lower values in Cohort II (Fig. 1 f). Again, this finding was present from T7 to L5. There were no significant differences regarding the age of subjects compared within each column in Fig. 1 (data not shown).
To analyze development of vertebral shape over time, a subgroup of Cohort II (n = 18) with a minimum follow-up of four years was measured at three different times: before GFSI surgery (av. age 7.5 + / − 1.6 yrs), after two years (av.age 9.5 + / − 1.6 yrs) and > 4 years (av. age 12.3 + / − 1.8 yrs) (Fig. 2). To evaluate different areas of the spinal column T4, T12 and L4 were compared. A significant increase in vertebral height was found for T12 (Fig. 2b) and L4 (Fig. 2c) for all time points and for T4 (Fig. 2a) after follow-up of four years. Vertebral depth did not show any significant differences at all three locations over time (Fig. 2d–f).
Finally, the morphology of the vertebra with regard to possible screw placement was analyzed. All vertebrae (Cohort II) displayed a typical shape with a small body and large spinal canal resulting in narrow pedicle diameter (Fig. 3). Pedicle diameters were measured from T1 to L5. No significant changes in pedicle diameter between Cohort I and II were detectable during the observation period (data not shown).
Vertebral morphology in SMA children with a-c and without d-f growth-friendly spinal implant (GFSI) treatment. The vertebrae shape in SMA children pre-treated with a GFSI is characterized by a small vertebral body [*]—especially in the lower thoracic and lumbar area—in comparison with untreated SMA children, narrow pedicles [arrowheads] in both groups and a large spinal canal [ +]. T4 thoracic vertebra 4; T12 thoracic vertebra 12; L5 lumbar vertebra 5
Discussion
Development of severe spinal deformity is one of the common sequelae of SMA type 1 and 2 children leading to a large C-shaped curve in the majority of patients. Conservative treatment with spinal orthosis may negatively affect lung function and therefore long-term survival [11] Therefore, early surgical intervention with either spinal fusion or GFSI is recommended [12]. However, spinal fusion below the age of ten years may lead to thoracic insufficiency syndrome with negative effects on long-term survival. Therefore, GFSI are currently the preferred method of treatment. Using this technique, the effect on vertebral morphology of distraction-based GFSI is poorly understood [13, 14].
Hasler et al. described vertebral lumbar height in a heterogenic pediatric population with spinal deformity treated by rib to pelvis vertebral expandable prosthetic titanium rib (VEPTR) constructs in comparison with rib to upper lumbar lamina instrumentations [6, 15]. The first group with instrumentation below the lumbar vertebral column (similar to the described implant construct in this paper) showed increased vertebral height over time and no change in vertebral depth. VEPTR instrumentation above the lumbar spine resulted in increased lumbar vertebral height and depth during GFSI treatment. The authors concluded that the apparent gains in height and reductions in depth are probably due to stress shielding secondary to spinal implants. In accordance with these data, we were able to demonstrate a clear increase in lumbar vertebral height and furthermore an increase in thoracic vertebral height in scoliotic SMA children treated with GFSI over time.
The data from Dimeglio and Canavese and from Brandner give a thorough insight in physiologic growth of the pediatric spine [16, 17]. In healthy children, the average growth velocity of the thoracic spine has been reported to be 13 mm/year from birth to five years, 7 mm/year from five to ten years and 11 mm/year during puberty. In the lumbar spine at age 10, most children have reached 90% of their final height. We found an average increase of 5.5 ± 3 mm in L4 from the beginning of GFSI treatment until spinal fusion (4.7 ± 0.5 years). These values are within the range of normal growth and are not increased as reported before [17]. In the thoracic spine, we observed a height increase of 2.8 ± 1.6 mm (T4) and 5.9 ± 2.1 mm (T12). With respect to previously published data, this increase within four years is well within physiologic ranges. Olgun et al. and others postulated an accelerated longitudinal growth up to double the physiological levels with growing rod treatment [18, 19]. Accelerated longitudinal growth, e.g., vertebral height, could not be found in our age-matched SMA patient groups. Therefore, our described data rather represent normal growth and increase in vertebral height and thus imply that natural growth is supported by GFSI with a 5 mm distraction rate every three months in SMA children.
The rate of distraction is under constant debate among pediatric spine surgeons. While traditional lengthening bears a procedure-related limit as most surgeons prefer six-monthly operative lengthening, with the advent of magnetically controlled devices more frequent lengthening is possible. Various distraction regimens have been proposed, and it remains unclear, at what rate these should be performed. We here applied 55 mm every three months with respect to the known natural growth rates, and our findings show that the morphology seems to follow this pattern.
Another general consideration is that the only description of vertebral shape under GFSI focuses on the lumbar region while the constructs mostly span rib to pelvis. To our knowledge, there are no comparable studies focusing on the thoracic changes of GFSI.
While our data are plausible but rather of descriptive character, one can only speculate on the underlying mechanism. Deformed vertebrae do react toward restoration of their normal shape by distraction as shown in small animal experiments [20, 21]. Lengthening causes strain or tension, and this proves to be a strong inducing factor. It remains unclear whether our findings result from true longitudinal growth of the vertebra or from a dynamic change of vertebral morphology. However, real growth seems to be more likely because of similar findings in age-matched data of Cohort I and Cohort II; the first group received the full axial loading without spinal instrumentation.
One of the key findings is that GFSI seem to negatively influence vertebral volume. Differences in volume CT data and morphology between GFSI-treated versus non-treated SMA children have a strong clinical impact. The vertebral body has a comparably small cross section in relation to that of the spinal canal and small pedicles along the whole spine. In general, pedicles in SMA patients were small with thin cortical walls which require caution when instrumentation is intended.
A secondary finding is that the volume underlies a gradual or almost linear change over time. Canavese et al. found that despite the well-recognized overall growth spurt during adolescence the growth of the thorax follows an almost linear pattern from age 5 to puberty [16]. These data were confirmed by our findings.
From the clinical point of view, a number of considerations can be discussed regarding possible consequences of our reported results. Loss of vertebral body volume in the lower thoracic and lumbar spine will most likely result in reduced spinal implant anchorage. However, this aspect is clearly related to bone mineral density (BMD) and SMA children will have osteoporosis in the majority of cases [22]. The effect of these findings on definite spinal fusion is still under evaluation. Instrumentation of every possible fusion level may be beneficial in order to avoid implant failure [23]. Lengthening with GFSI could also affect stability by distracting the uncovertebral joints. Again, no clinically relevant problems were seen in the GFSI SMA group after removal of implants prior to definitive spinal fusion, but this effect remains unclear.
Limitations of the study are (a) the homogeneous population of SMA children. It is unclear if these data can be transferred to other patient groups; (b) radiographic measurements, such as vertebral height and depth, are dependent upon several factors such as the focus of the X-ray beam, projection artifacts or vertebral body rotation [7, 8]. Also, (c) vertebral body morphometrics are highly dependent on bone mineral density. In this study, no data on osteoporosis are integrated in the analysis. Additionally, the role of GFSI on intervertebral disk space remains unclear and was not within the focus of the study.
Conclusion
In summary, we describe a previously unrecognized characteristic influence of GFSI in a prospectively evaluated cohort of scoliotic children with SMA. To our knowledge, for the first time, 3D CT volume rendering is used to describe morphologic vertebral body changes related to this specific surgical technique. By using volume rendering, we were able to measure growth-related parameters in the thoracic spine, which have not been previously described. The findings add to knowledge about an increasingly popular treatment method for scoliosis in the growing child. Further investigations will be necessary to fully understand the biological changes, i.e., characteristics of bone growth that lead to loss of volume and decreased vertebral depth while vertebral height follows the usual growth pattern.
References
Mercuri E, Finkel RS, Muntoni F et al (2018) Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 28:103–115. https://doi.org/10.1016/j.nmd.2017.11.005
Shorrock HK, Gillingwater TH, Groen EJN (2018) Overview of current drugs and molecules in development for spinal muscular atrophy therapy. Drugs 78:293–305. https://doi.org/10.1007/s40265-018-0868-8
Perrone M, Orr R, Hing W et al (2018) The impact of backpack loads on school children: a critical narrative review. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15112529
Hell AK, Groenefeld K, Tsaknakis K et al (2018) Combining bilateral magnetically controlled implants inserted parallel to the spine with rib to pelvis fixation: surgical technique and early results. Clin Spine Surg 31:239–246. https://doi.org/10.1097/BSD.0000000000000614
Lorenz HM, Badwan B, Hecker MM et al (2017) Magnetically controlled devices parallel to the spine in children with spinal muscular atrophy. JB JS Open Access 2:e0036. https://doi.org/10.2106/JBJS.OA.17.00036
Hasler CC, Studer D, Büchler P (2015) Metamorphosis of human lumbar vertebrae induced by VEPTR growth modulation and stress shielding. J Child Orthop 9:287–293. https://doi.org/10.1007/s11832-015-0677-5
Allaire BT, DePaolis Kaluza MC, Bruno AG et al (2017) Evaluation of a new approach to compute intervertebral disc height measurements from lateral radiographic views of the spine. Eur Spine J 26:167–172. https://doi.org/10.1007/s00586-016-4817-5
Frobin W, Brinckmann P, Biggemann M et al (1997) Precision measurement of disc height, vertebral height and sagittal plane displacement from lateral radiographic views of the lumbar spine. Clin Biomech (Bristol, Avon) 12(Suppl 1):S1–S63. https://doi.org/10.1016/s0268-0033(96)00067-8
Wagner L, Braunschweig L, Eiffert H et al (2018) Detection of bacteria colonizing titanium spinal implants in children. Surg Infect (Larchmt) 19:71–77. https://doi.org/10.1089/sur.2017.185
Klinder T, Ostermann J, Ehm M et al (2009) Automated model-based vertebra detection, identification, and segmentation in CT images. Med Image Anal 13:471–482. https://doi.org/10.1016/j.media.2009.02.004
Tangsrud SE, Carlsen KC, Lund-Petersen I, Carlsen KH (2001) Lung function measurements in young children with spinal muscle atrophy; a cross sectional survey on the effect of position and bracing. Arch Dis Child 84:521–524. https://doi.org/10.1136/adc.84.6.521
Lenhart RL, Youlo S, Schroth MK et al (2016) Radiographic and respiratory effects of growing rods in children with spinal muscular atrophy. J Pediatr Orthop. https://doi.org/10.1097/BPO.0000000000000867
Campbell RM, Hell-Vocke AK (2003) Growth of the thoracic spine in congenital scoliosis after expansion thoracoplasty. J Bone Joint Surg Am 85-A:409–420
Mehta HP, Snyder BD, Callender NN et al (2006) The reciprocal relationship between thoracic and spinal deformity and its effect on pulmonary function in a rabbit model: a pilot study. Spine 31:2654–2664. https://doi.org/10.1097/01.brs.0000244613.66055.b6
Campbell RM (2013) VEPTR: past experience and the future of VEPTR principles. Eur Spine J 22(Suppl 2):S106-117. https://doi.org/10.1007/s00586-013-2671-2
Canavese F, Dimeglio A (2013) Normal and abnormal spine and thoracic cage development. World J Orthop 4:167–174. https://doi.org/10.5312/wjo.v4.i4.167
Brandner ME (1970) Normal values of the vertebral body and intervertebral disk index during growth. Am J Roentgenol Radium Ther Nucl Med 110:618–627. https://doi.org/10.2214/ajr.110.3.618
Ridderbusch K, Rupprecht M, Kunkel P et al (2017) Preliminary results of magnetically controlled growing rods for early onset scoliosis. J Pediatr Orthop 37:e575–e580. https://doi.org/10.1097/BPO.0000000000000752
Olgun ZD, Ahmadiadli H, Alanay A, Yazici M (2012) Vertebral body growth during growing rod instrumentation: growth preservation or stimulation? J Pediatr Orthop 32:184–189. https://doi.org/10.1097/BPO.0b013e3182471915
Mente PL, Aronsson DD, Stokes IA, Iatridis JC (1999) Mechanical modulation of growth for the correction of vertebral wedge deformities. J Orthop Res 17:518–524. https://doi.org/10.1002/jor.1100170409
Aronsson DD, Stokes IA, Rosovsky J, Spence H (1999) Mechanical modulation of calf tail vertebral growth: implications for scoliosis progression. J Spinal Disord 12:141–146
Vai S, Bianchi ML, Moroni I et al (2015) Bone and spinal muscular atrophy. Bone 79:116–120. https://doi.org/10.1016/j.bone.2015.05.039
Weiser L, Huber G, Sellenschloh K et al (2017) Insufficient stability of pedicle screws in osteoporotic vertebrae: biomechanical correlation of bone mineral density and pedicle screw fixation strength. Eur Spine J 26:2891–2897. https://doi.org/10.1007/s00586-017-5091-x
Acknowledgements
The authors (KL, LB, KT, HML and AKH) are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD).
Funding
Open Access funding enabled and organized by Projekt DEAL.. No funding was obtained for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Formal consent was required from all study participants. All authors have seen and approved the final version of the manuscript being submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lippross, S., Grages, A., Lueders, K.A. et al. Vertebral body changes after continuous spinal distraction in scoliotic children. Eur Spine J 30, 1928–1934 (2021). https://doi.org/10.1007/s00586-021-06775-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-021-06775-7