Abstract
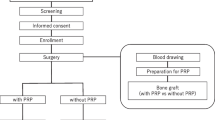
Over the last few years, some hemocomponents have been used advantageously in clinical neurosurgical practice, not systemically via transfusion but topically as a sealant (fibrin glue). This has diverted the attention of many authors to the role of platelets in the healing process. The combination of hyper-concentrated platelets and fibrin glue (fibrinogen, XIII factor, fibronectin) with activated thrombin produces a platelet gel that can be easily applied to “difficult” wounds. This topical use of hemocomponents has gained an important role in regenerative medicine. The authors have considered the possibility of using a preparation with a high autologous platelet concentration applied in addition to autologous bone during vertebral postero-lateral fusion. The aim of the procedure is to induce a higher rate of vertebral fusion. Between November 2007 and November 2008, 14 patients (9 men and 5 women, mean age 58.9) underwent laminectomy, vertebral stabilization and postero-lateral fusion. The number of vertebral levels involved in stabilization was: 1 in 2 patients, 2 in 5 patients, 3 in 5 patients, 4 in 1 patient and 5 in 1 patient. Platelet gel was obtained by taking 16 ml of peripheral venous blood from the patient. For this procedure two patented test tubes were used for each patient, with a capacity of 8 m each. These make up the REGEN-THT® (Thrombocyte Harvesting Tube) system that makes it possible to obtain 8 ml of autologous platelet gel in 40–45 min. The addition of Ca gluconate and ethanol at 95% makes it possible to obtain a preparation of plasma rich in platelets and activated thrombin with a platelet concentration five times superior to the haematic one. The platelet gel is combined with fragments of autologous bone and synthetic bone during surgical operation. To allow a comparative assessment of the degree of fusion achieved with and without application of the platelet preparation in each patient, it was arbitrarily decided to use it in only one half of the operative field. All patients underwent serial CT scans 3 and 6 months after surgery as well as plain X-rays to evaluate bone fusion. The reconstructed CT images, especially in sagittal and axial planes, permitted an evaluation of the degree of vertebral fusion and “bone growth”. The fusion rate was calculated measuring the increment of bone density on CT images, by means of an evaluation of the ROI (HU) in the newly formed bone, and comparing bone density within the bone callus formed by autologous and synthetic bone alone in the one to which the platelet preparation had been added. A good rate of fusion was observed in all patients. Furthermore, a comparative analysis of ROI at 3 and 6 months after surgery demonstrated a high increase in the fusion rate during the first 3 months after surgery. After 6 months the differences in ROI between the two sides had balanced out. However, at 6-month follow-up examination, bone density in the half of the surgical field in which platelet gel had been added to autologous–heterologous bone was higher in comparison to the contralateral one. Bony neoformation after posterior-lateral arthrodesis is well-evident 3 months after surgery and usually continues gradually for the following 18–24 months. The autologous platelet preparation used seems to accelerate bony deposition and to promote tissue healing, increasing bone density at the level of posterior–lateral arthrodesis. Moreover, this preparation has low production costs and is easy to apply.


Similar content being viewed by others
References
Bose B, Balzarini M (2002) Bone graft gel: autologous growth factors used with autograft bone for lumbar spine fusion. Adv Ther 19:170–175
Brown CW, Orme TJ, Richardson HD (1986) The rate of pseudoarthrosis (surgical non-union) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine 11:942–943
Canalis E et al (1985) Effect of growth factors on bone cell replication and differentiation. Clin Orthop 193:246–263
Canalis E et al (1989) Effects of platelet derived growth factor on bone formation in vitro. J Cell Physiol 140:530–537
Carreon LY et al (2005) Platelet gel (AGF) fails to increase fusion rates in instrumented posterolateral fusions. Spine V 30(9):E243–E246
Centrella M et al (1986) Human platelet derived transforming growth factor-b stimulates parameters of bone growth in fetal rat calvariae. Endocrinology 119:2306–2312
Centrella M et al (1989) Platelet derived growth factor enhances deoxyribonucleic acid and collagen synthesis in osteoblast-enriched cultures from fetal rat parietal bone. Endocrinology 125:13–19
Dohan E, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte-and platelet-rich fibrin (L-PRF). Trends Biotechnol 27(3):158–167
Eppley B, Woodell J, Higgins J (2004) Platelet quantification and growth factor analysis from platelet rich plasma (PRP): implications in wound healing. Plastic Reconstruct Surg 114:1502–1508
Glassman SD, Anagnost SC, Parker A (2000) The effect of cigarette smoking and smoking cessation on spinal fusion. Spine 25:2608–2615
Hannon T, Polston G, Pekarske W (1996) Determination of platelet yields from platelet rich plasma for autotransfusion machines. Anesth Analg 88:104–109
Hee H, Madj M, Holt R, Myers L (2003) Do autologous growth factors enhance transforaminal lumbar interbody fusion? Eur Spine J 12:400–407
Howes R et al (1988) Platelet derived growth factor enhances demineralised bone matrix induced cartilage and bone formation. Calcif Tissue Int 42:34–38
Jenis LG, Banco RJ, Kwon B (2006) A prospective study of autologous growth factors (AGF) in lumbar interbody fusion. Spine J 6:14–20
Kasperk CH et al (1990) Interactions of growth factors present in bone matrix with bone cells effects on DNA synthesis and alkaline phosphatase. Growth Factors 3:147–158
Kevy S, Jacobson M (2004) Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corp Technol 36:28–35
Lowery GL, Kulkarny S, Pennisi AE (1999) Use of autologous growth factors in lumbar spinal fusion. Bone Aug Suppl 25(2):47S–50S
Marx RE et al (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
Marx RE et al (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62(4):489–496
Noda M et al (1998) In vivo stimulation of bone formation by transforming growth factor B. Endocrinology 124:2991–2994
Oprea WE, Karp JM, Hosseini MM, Davies JE (2003) Effect of platelet releasate on bone cell migration and recruitment in vitro. J Craniofac surg 14(3):292–300
Pfeilschifter J et al (1990) Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet derived growth factor, and transforming growth factor B. Endocrinology 127:69–75
Slater M et al (1995) Involvement of platelets in stimulating osteogenic activity. J Orthop Res 13:655–663
Vaccaro AR, Sharan AD, Tuan RS et al (2001) The use of biologic materials in spinal fusion. Orthopaedics 24:191–197
Van Den Dolder J, Mooren R et al (2006) Platelet rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng 12 (11)
Weiner BK, Wolker M (2003) Efficacy of autologous growth factors in lumbar intertransverse fusion. Spine 28(17):1968–1971
Zimmermann R, Jakubietz R, Jakubietz M et al (2001) Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 41:1217–1224
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Landi, A., Tarantino, R., Marotta, N. et al. The use of platelet gel in postero-lateral fusion: preliminary results in a series of 14 cases. Eur Spine J 20 (Suppl 1), 61–67 (2011). https://doi.org/10.1007/s00586-011-1760-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-011-1760-3