Abstract
Mercury (Hg) pollution of soils is a critical environmental problem. To rehabilitate Hg contaminated soils, arbuscular mycorrhizal (AM) fungi-based phytoremediation may be supportive, yet the functional potential of AM fungi in response to Hg exposure is unclear. In a greenhouse experiment, we assessed the response of Medicago truncatula (Hg tolerance index (TI), Hg partitioning) to different Hg concentrations [0 (Hg0), 25 (Hg25), 50 (Hg50) µg g−1] in treatments with (AM) and without (NM) inoculation of Rhizophagus irregularis. Additionally, zinc (Zn) uptake and the expression of two Zn transporter genes (ZIP2, ZIP6) were examined because Zn is an essential element for plants and shares the same outer electronic configuration as Hg, implying potential competition for the same transporters. The results showed that AM plants had a higher TI than NM plants. Plant roots were identified as dominant Hg reservoirs. AM inoculation reduced the root Hg concentration under Hg50 compared to the NM treatment. There was an interaction between Hg treatment and AM inoculation on Hg stem concentration, i.e., at Hg25, AM inoculation decreased the Hg translocation from roots to stems, while Hg translocation was increased at Hg50 compared to the NM treatment. Zn acquisition was improved by R. irregularis. The negative relationship between Hg and Zn concentrations in the roots of AM and NM plants implied potential competition for the same transporters, although the expression of Zn transporters was upregulated by AM inoculation at all Hg levels. In conclusion, this baseline study demonstrated that R. irregularis may play an important role in Hg tolerance of M. truncatula, suggesting its potential for Hg-contaminated phytoremediation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mercury (Hg) is a very toxic heavy metal (HM), ranked as the third most hazardous substance on earth (ATSDR 2019). Because of its high mobility and persistence in the environment, Hg has been recognized as a global pollutant and major threat to human health (Driscoll et al. 2013). Pollution with Hg occurs naturally by weathering and to a large extent through anthropogenic activities. These include artisanal gold mining, stationary combustion of coal, nonferrous metals production, and cement production. Among these, artisanal gold mining is the world’s largest source of anthropogenic Hg emission (EPA 2018) and is done in many regions around the world (Wang et al. 2022). This practice often is uncontrolled and aggravates the distribution of Hg in terrestrial ecosystems, which may affect the growth of food crops, thereby compromising food safety (Gworek et al. 2020).
Decontamination of Hg polluted soils is a major concern in environmental legislation and food production. Phytoremediation, a plant-based technology, offers a green and sustainable solution to remediate Hg contaminated soils. Phytoextraction (facilitating Hg translocation to plant stems) and phytostablization (facilitating Hg storage in plant roots, while preventing Hg translocation to plant stems) are two popular approaches of phytoremediation of soil Hg contamination (Bhat et al. 2022). Plants, however, may suffer from negative effects of Hg toxicity, which limits plant growth and even threatens plant survival. To counteract such negative impacts on plant performance in the process of soil remediation, plants can be inoculated with competent soil microorganisms, specifically arbuscular mycorrhizal fungi (AMF) (Ferrol et al. 2016).
AMF are ubiquitous in terrestrial environments and form intimate relationships with the majority of vascular plants (Smith and Read 2008), reflecting the strategic importance of plant-AMF interactions for environmental adaptation (Wang and Qiu 2006). Several studies reported that certain arbuscular mycorrhizal (AM) fungal species facilitate HM transport to aboveground plant organs (phytoextraction) (Weissenhorn et al. 1995; Fiqri et al. 2016; Singh et al. 2019). Other studies, however, revealed that AM fungal species inhibit HM translocation by various means (Shabani et al. 2016; Chamba et al. 2017; Salazar et al. 2018). These include the binding of HM in AM fungal hyphae and glomalin, or sequestering HM in fungal structures (arbuscules, vesicles, vacuoles). With this, the transport of HM to the plant stem is prevented (phytostablization) (Garg and Singh 2018; Motaharpoor et al. 2019). The extent of HM uptake and the subsequent translocation in planta further depends on the HM concentration in soils, even in the presence of the same AM fungal species (Huang et al. 2017).
Hg exists in three forms, i.e., elemental Hg, inorganic Hg (Hg1+, Hg2+) and organic Hg (Beckers and Rinklebe 2017). In soils, the predominant form is Hg2+ (Beckers and Rinklebe 2017; Kumari et al. 2020). Uptake of Hg2+ via roots is facilitated by an active process (Esteban et al. 2008; Wang et al. 2014; Ma et al. 2021), yet no precise membrane transporters involved in root Hg2+ have been identified. It is generally accepted that HM enter roots via nutrient transporters (Manoj et al. 2020) because of the structural similarity of HM with essential nutrients. For example, arsenate (AsO43−) is taken up by the same transporters as phosphate (PO43−) (Meharg and Hartley-Whitaker 2002) and arsenite shares transporters with silicic acid (Ma et al. 2008). Cadmium (Cd2+) uptake by plants is facilitated via zinc (Zn) protein carriers (Kaur and Garg 2018). Such pertinent information is lacking for Hg, however, despite that Hg2+, like Cd2+, shares the same outer electronic configuration as Zn2+ (Jensen 2003). Competition between Hg2+ and Zn2+ was observed in bacteria upon addition of Zn to a Hg-containing growth solution. These findings imply that Hg and Zn share affinity for the same transporters (Schaefer et al. 2014; Szczuka et al. 2015). Zn is an essential micronutrient for plants, playing vital roles in cellular and physiological functions (Fariduddin et al. 2022). Zn has a low mobility in soil solution, whereby its uptake is diffusion-limited (Lehmann et al. 2014). AMF have been recognized to play a key role in facilitating Zn tissue concentration (Ruytinx et al. 2020). These results highlight the importance of investigating the interplay between Zn and Hg uptake in relation to mycorrhizal colonization, which is fundamental to understand the mechanisms of Hg uptake and accumulation in mycorrhizal plants. Such advanced knowledge would help to optimize AMF-supported plant performance in phytoremediation.
Among the involved transporters of Zn into the cytoplasm, the zinc-iron-regulated transporter (ZRT-IRT) family, called ZIP, mostly has been studied. In Medicago truncatula, four ZIP transporters—ZIP1, ZIP2, ZIP5, and ZIP6 facilitating the transport of Zn2+—have been verified in yeast complementation assays (Burleigh et al. 2003; Stephens et al. 2011). Recent studies showed that only two of them are influenced by mycorrhizal colonization, yet under contrasting Zn conditions. Namely, ZIP6 was up-regulated at deficient and sufficient soil Zn concentrations, while ZIP2 was up-regulated at toxic Zn concentrations (Watts-Williams et al. 2017).
In this study, we used the model legume Medicago truncatula inoculated with the AM fungus Rhizophagus irregularis to gain fundamental insights into the underlying mechanisms of Hg uptake and accumulation by mycorrhizal plants. The aims of this study were to (1) examine the effect of R. irregularis on biomass and Hg accumulation of M. truncatula under Hg exposure; (2) determine the translocation strategies of Hg in roots, stems and leaves of M. truncatula associated with R. irregularis; and (3) investigate the effect of R. irregularis on Zn nutrient uptake and Zn transporters (ZIP2, ZIP6) under Hg exposure.
Materials and methods
Preparation of biological materials
Medicago truncatula cv. Jemalong A17 seeds were scarified in 90% sulfuric acid for 7.5 min. Seeds were washed eight times with cold water to remove the acid, followed by 90 s surface sterilization in 3% active chlorine solution (sodium hypochlorite solution) (Garcia et al. 2006). The chlorine solution was removed by rinsing of the seeds in sterile water for 5–6 times. Sterilized seeds were soaked in sterile water overnight under darkness at room temperature (20 °C). Then, the seeds were stratified in water-agar plates (0.8% (w/v)) for 24 h at 4 °C and germinated at 20 °C for 2 days in the dark. After germination, seeds were exposed to light for 2 days to initiate chlorophyll development. The AM fungus Rhizophagus irregularis (QS81) was provided by INOQ GmbH (Schnega, Germany). The inoculum of R. irregularis was prepared from arbuscular mycorrhizal root fragments of Trifolium partensis produced in sand/vermiculite 35/65 v/v in year 2019. The product was filtered through a 425 µm mesh (grade II) and finally contained 100 million propagules kg−1 powder (as vesicles and spores according to the manufacturer). Prior to use, the AM fungal propagules were stored in an air-dried, well-ventilated, and dark environment.
Experiment design and conditions
Sand quartz was twice autoclaved (121 °C/2 h) over a 2-day interval. For the AM fungus inoculated treatment (AM), 50 ml (80 g) sterilized sand were mixed with 25 mg (a ratio of 0.5 g L−1 substrate) Osmocote Exact Mini 3–4 months (NPK 15:3.9:9.1 + 1.2 Mg + trace elements, ICL Specialty Fertilizers, UK) (Senovilla et al. 2020), and 160 mg (a ratio of 3.2 g L−1 substrate) AM fungus inoculum (Mercy et al. 2017) was added to each plastic pot (5 cm of height, 4 cm of width and with 3 holes in the bottom). For the negative control without inoculum (NM), the same amount of sand mixed with 25 mg Osmocote Exact Mini was filled into each plastic pot. Then, one seedling (5 days) was transferred to each pot. Plants were grown in the greenhouse from 19 June to 17 August, 2021. Plants were maintained at an average temperature of 29.4 °C and an average relative humidity of 51% under natural light conditions (Phytotechnikum research station, University of Hohenheim, Stuttgart, Germany). Plants were watered daily with 5-ml tap water which did not drain from the pots. After 3 weeks, when plants showed vigorous growth, 5 ml HgCl2 solution at concentrations of 25 µg g−1 or 50 µg g−1 were added to each Hg treatment pot once, respectively. The reference pots without Hg application were treated with sterile water in equivalent volume. The Hg treatment was assigned to Hg0, Hg25, and Hg50. The experiment was a 2 × 3 complete factorial design, comprising 5 replications per treatment arranged in a randomized block design. The experiment was performed for 5 weeks after Hg additions until destructive harvest.
Plant harvest
At the end of experiment (8 weeks), the roots were quickly washed with tap water and separated into 3 parts. One sub-sample (100 mg) of fresh root was immediately flash frozen in liquid nitrogen and stored at −80 °C for RNA isolation. The second sub-sample also was stored at −80 °C for AM root colonization observation. The remaining roots as well as stem and leaf biomass were dried at room temperature until weight stability to determine plant biomass weight.
The tolerance index (TI) was calculated (Eq. 1) to reflect the ability of the host plant to grow in the presence of different Hg concentration (Huang et al. 2017). There, mt is the total dry biomass of the plant growing in each pot; mc denotes the average total dry biomass of the plants growing in pots without Hg under NM and AM treatment, separately.
Determination of Hg and Zn
Hg and Zn concentrations were determined separately for roots, stems, and leaves. Each air-dried sample was milled with a centrifugal mill (Retsch GmbH, Haan, Germany) equipped with a titanium rotor and a ring sieve. Samples (0.2 g) were moistened with 1 ml of deionized H2O and digested in 2.5 ml 69% HNO3 in an UltraCLAVE III microwave heated digestion unit (MLS-MWS GmbH, Leutkirch, Germany). After digestion, the solutions were filled up to 10 ml with deionized H2O. Hg concentration was analyzed via a NexION 300 × inductively coupled plasma mass spectrometry (ICP-MS) (PerkinElmer LAS GmbH, Rodgau, Germany), and Zn concentration was analyzed by an Agilent5100 inductively coupled plasma optical emission spectrometry (ICP-OES) (Agilent Technologies GmbH, Waldbronn, Germany) (Core Facility, University of Hohenheim, Stuttgart, Germany).
Mycorrhizal colonization
Frozen roots stored for AMF observation were cut into 1 cm segments and cleared using 10% NaOH in a 70 °C water bath for 45 min and then soaked in 1% HCl for 1 min at room temperature. The roots were stained in 2% PARKER QUINK blue ink (Yon et al. 2015) in a 70 °C water bath for 30 min. The roots were rinsed with tap water until the water appeared transparent and then were stored in a lactoglycerol solution (v:v:v–1:1:1-latic acid:glycerol:H2O). Thirty fragments were randomly selected, placed on a slide, and checked under a light microscope for intraradical AM structures (Trouvelot et al. 1986).
RNA isolation and quantitative RT-PCR
Total RNA was isolated from 100 mg root sub-samples (RNeasy® plant Mini Kit, QIAGEN GmbH, Germany). RNA integrity was checked by gel electrophoresis, following quantification of RNA by Nanodrop 2100 (Thermo Fisher Scientific). The cDNA was synthesized from 500 ng of RNA using the QIAGEN QuantiTect® Reverse Transcription Kit (QIAGEN GmbH) including a genomic DNA elimination step. Quantification of the expression of ZIP2, ZIP6, and Ri-tubulin genes was conducted with 1 µl of 10 × diluted cDNA in a 20 µl reaction using gene-specific primers and SYBR® green PCR Master Mix in the StepOnePlus™ Real-Time PCR system (Applied Biosystems, USA). The RT-PCR settings were 94 °C for 5 min, then 94 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s for 40 cycles, followed by generation of a dissociation curve. MtASPP and β-actin were selected as housekeeping genes using NormFinder (Mestdagh et al. 2009). Gene expressions were normalized to the geometric mean of the two selected housekeeping genes (Vandesompele et al. 2002). Table S1 displays the sequences of the forward and reverse primers used in this study.
Statistical analysis
All statistical analyses were performed in R version 4.0.3. Homogeneity and normality of data were checked with Levene’s test and Shapiro–Wilk test, respectively. Data, which did not meet the criteria, were Box-Cox transformed (Box and Cox 1964). Values presented in figures are non-normalized data. Response variable data were subjected to two-way analysis of variance (ANOVA), with the factors “Hg treatment” and “AM inoculation.” Following two-factor ANOVA, the means of AM fungus inoculation (AM) and non-inoculated control (NM) were compared separately at each Hg level (Hg0, Hg25, Hg50) using Student’s t-test (5%). For response variables, for which no AM fungus data could be recorded (i.e., mycorrhizal colonization and α-tubulin expression of R. irregularis in NM treatments), one-way ANOVA along with Tukey’s honestly significant difference (HSD) was used considering Hg treatment as the only factor. A correlation matrix among Hg concertation and Zn concentration in each plant part, as well as ZIP transporter gene expression, was calculated for AM and NM treatments, respectively. Spearman’s method was used, and P value was adjusted with a Bonferroni correction.
Results
Plant Hg tolerance and mycorrhizal colonization
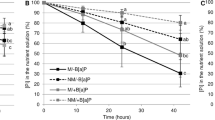
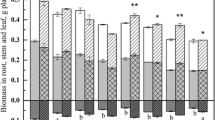
Overall, inoculation with Rhizophagus irregularis (AM plants) conferred higher Hg tolerance to Medicago truncatula compared to non-inoculated controls (NM plants) (P < 0.001) (Table 1). This effect was significant for Hg25 (P < 0.01) (Fig. 1). The results of dry biomass in each part (leaves, stems, and roots) and total biomass are shown in Table S2. Leaf necrosis in NM plants under Hg50 treatment was observed.
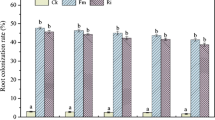
Under Hg0, Hg25, and Hg50, frequencies of mycorrhizal colonization of 42.7%, 55.8%, and 34%, respectively, were determined but did not differ significantly (P > 0.05) (Fig. 2a, Table 1). Pictures of colonization are shown in Fig S1. The expression of the α-tubulin gene in mycorrhizal roots of AM plants followed the same trend (Fig. 2b). Non-inoculated roots (NM plants) were free of mycorrhizal colonization, as confirmed by lack of α-tubulin gene expression in their roots.
Percentage of mycorrhizal colonization a and relative expression of AM fungal biomass marker gene α-tubulin b in roots of Medicago truncatula plants inoculated with Rhizophagus irregularis and grown at different Hg concentrations. Values present mean ± SE (n = 5). There were no significant effects of Hg treatment on both mycorrhizal colonization and relative expression of α-tubulin (see Table 1 for detailed ANOVA analysis)
Concentration and accumulation of Hg
Figure 3 shows the Hg concentration in different plant parts (leaves, stems, roots). AM inoculation reduced the Hg concentration in leaves at all Hg concentrations compared to NM plants (P < 0.001) (Table 1, Fig. 3a). Hg concentrations in leaves at different Hg treatments were similar (Fig. 3a). There was no Hg contamination detected in the control substrate (Hg0) after the experiment (Fig. S2). Therefore, Hg accumulation in leaves likely results from absorbance of atmospheric Hg, because all plants were grown in the same compartment in the greenhouse. There was a significant interaction between Hg treatment and AM fungus inoculation for Hg concentration of stems (P < 0.001) (Table 1). The Hg stem concentration under Hg25 was lower in AM plants than in NM plants (P < 0.05) (Fig. 3b). Conversely, under Hg50, the Hg stem concentration was higher in AM plants than in NM plants (P < 0.0001) (Fig. 3b). For roots, there was a significant interaction between Hg treatment and AM fungus inoculation (P < 0.001) (Table 1), which was most prominent under Hg50 (P < 0.05) (Fig. 3c). Concerning Hg contents in different plant parts, AM fungus inoculation significantly reduced the Hg content in leaves compared to NM plants (P < 0.001) (Table 1). There also was a significant interaction between Hg treatment and AM inoculation for Hg stem content (P < 0.01) (Table 1). For Hg root content, only Hg treatment had a significant main effect (P < 0.001) (Table 1). Figure 4 shows the percentage of Hg content in each plant part, substantiating the root as prominent plant tissue for Hg accumulation under Hg treatment (Fig. 4).
Mercury (Hg) concentration in leaves a, stems b, and roots c of non-mycorrhizal (NM) and mycorrhizal (AM) plants exposed to three different Hg levels (Hg0, Hg25, Hg50). Values present mean ± SE (n = 5). Asterisks (*) denote a significant mean difference between NM and AM treatments, as measured by the t-test (5%). Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant (P > 0.05)
Zn concentration and Zn transporter gene expression
There was a significant interaction between Hg treatment and AM fungus inoculation on Zn concentration in leaves (P < 0.05) (Table 1). Specifically, AM plants showed higher Zn concentrations than NM plants under Hg25 and Hg50 (P < 0.05) (Fig. 5a). AM fungus inoculation increased Zn concentrations in stems compared to NM plants (P < 0.001) (Table 1, Fig. 5b). In contrast, AM plants showed lower Zn concentrations in roots than NM plants (P < 0.01) (Table 1; Fig. 5c). However, considering Zn content in different plant parts, AM inoculation only had a significant effect on Zn content in stems (P < 0.05) (Table 1), by increasing the percentage of Zn content in stems when inoculated with AM fungus (Fig. 6). AM fungal inoculation significantly upregulated the expression of ZIP2 (P < 0.01) and ZIP6 (P < 0.001) genes, irrespective of Hg treatment (Table 1, Fig. 7).
Zinc (Zn) concentration in leaves a, stems b, and roots c of non-mycorrhizal (NM) and mycorrhizal (AM) plants exposed to three different mercury (Hg) levels (Hg0, Hg25, Hg50). Values present mean ± SE (n = 5). Asterisks (*) denote a significant mean difference between NM and AM treatments, as measured by the t-test (5%). Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant (P > 0.05)
The relative gene expression of MtZIP2 a and MtZIP6 b in non-mycorrhizal (NM) and mycorrhizal (AM) plants exposed to three different mercury (Hg) levels (Hg0, Hg25, Hg50). Values present mean ± SE (n = 5). Asterisks (*) denote a significant mean difference between NM and AM treatments, as measured by the t-test (5%). Significance levels: *P < 0.05; **P < 0.01; ***P < 0.001
Correlations between Hg and Zn concentrations in roots
Correlation matrices among measured parameters were calculated for NM and AM treatments separately (Table S3 and Table S4). Hg concentration in stems was negatively correlated with Zn concentration in roots of the NM plants (r = −0.74; P < 0.05), and Hg concentration in roots was negatively correlated with Zn concentration in stems under AM treatment (r = −0.70; P < 0.05). Interestingly, Hg concentration in roots was negatively correlated with Zn concentration in roots for both NM and AM treatments (r = −0.71; r = −0.70, respectively; P < 0.05).
Discussion
Effects of Rhizophagus irregularis on Hg tolerance index and Hg partitioning
Numerous studies have indicated that AMF inoculation could alleviate HM stress of plants (Garg and Singh 2018; Motaharpoor et al. 2019; Molina et al. 2020). In line with this, our results showed a positive effect of Rhizophagus irregularis on Hg tolerance of Medicago truncatula, suggesting the potential of using AM fungi in Hg phytoremediation. Furthermore, our results showed the effects of R. irregularis on Hg partitioning in each plant part (leaves, stems and roots). There was a significant reduction of Hg concentration and content in leaves of AM plants compared to NM plants, indicating that the strain of R. irregularis was able to protect leaves from Hg uptake. Assad et al. (2016) demonstrated that Hg uptake by leaves is exclusively caused through the atmospheric pathway. This also was indicated in our study in which leaf Hg concentration was similar under different Hg concentration treatments. Thus, the reduction of Hg concentration in leaves might be attributed to the regulation of stomatal closure by R. irregularis. This possibility deserves further investigation. Wang et al. (2017) showed that stomatal closure is part of the protective strategy initiated by R. irregularis under exposure to cadmium.
Additionally, a significant interaction between Hg treatment and AM inoculation on Hg stem concentration and content was detected in our study, indicating that the effect of R. irregularis on Hg translocation to stems was dependent on Hg concentration in the substrate. Prior work showed that maize associated with Funneliformis mosseae (formerly Glomus mosseae) did not influence Hg shoot concentration compared to non-inoculated plants exposed to Hg concentrations of 2 and 4 mg kg−1 (Yu et al. 2010). Recent studies have demonstrated, however, that Glomus sp. associated with maize facilitated Hg uptake and its translocation from roots to shoots at a Hg concentration of 50 mg kg−1 (Kodre et al. 2017; Debeljak et al. 2018). Together, these results indicate that the role of AM fungal species in Hg translocation in plants is likely dependent on the Hg concentration in the substrate, as was corroborated by our study.
The observed increase of Hg concentrations in roots with increasing Hg levels was consistent with other reports. For example, Hg concentration in both lupin and maize roots showed a hyperbolic pattern with increasing Hg additions in the growth solution (Esteban et al. 2008; Yu et al. 2010). Furthermore, AM inoculation reduced Hg concentration at Hg50, indicating a protective role of R. irregularis under high Hg concentration. This reduction was generally attributed to the role of AM fungal structures, e.g., binding with hyphae and sequestration into vacuoles. However, this may not be the case in the present study because such a reduction was not observed under Hg25. There is no difference in Hg root concentrations between NM and AM treatments under Hg25, indicating the capacity of root Hg accumulation of M. truncatula. Within this capacity, plants can detoxify Hg by themselves (Kumar et al. 2017), but plants may need support from microbes (in this case R. irregularis) at high concentrations, as it was shown under Hg50. Accordingly, further work will be necessary to precisely investigate the functions of the same AM fungal species on different plant species exposed to different Hg concentrations.
Effects of Rhizophagus irregularis on Zn under Hg exposure
Generally, AM inoculation exerts a positive effect on the transport of elemental nutrients (Smith and Read 2008). In our study, AM inoculation increased Zn concentration in aboveground plant parts exposed to Hg, a finding in line with other studies (Debeljak et al. 2018; Saboor et al. 2021). Moreover, AM fungus inoculation upregulated the expression of Zn transporter genes (ZIP2, ZIP6), independent of Hg levels in the substrate. This indicates that Hg uptake by roots might not have been related to these two transporters in our study. This also was indicated by the absence of correlations between Hg root concertation and the two assayed ZIP transporter genes. Nevertheless, negative relationships between Hg and Zn concentrations in the roots of both AM and NM plants were found, implying potential competition between both elements for the same transporters. Our results suggest that Hg did not impair the positive regulation of Zn nutrient and its transporter genes by R. irregularis, which may have contributed to Hg tolerance because Zn plays a vital role in the reduction of the oxidative stress caused by Hg (Calgaroto et al. 2011). In order to fully understand the relationship between both Zn and Hg, further experiments with different Zn concentrations in the substrate and studies on other Zn-related transporter genes are needed.
Conclusions
Our results showed that the regulatory role of R. irregularis in Hg accumulation and translocation from roots to stems in Medicago truncatula is dependent on the concentration of Hg in the substrate. Additionally, a positive effect of R. irregularis on Hg tolerance of M. truncatula along with improvement of Zn nutrient status and upregulation of Zn transporter genes (ZIP2, ZIP6) was found.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Assad M, Parelle J, Cazaux D, Gimbert F, Chalot M, Tatin-Froux F (2016) Mercury uptake into poplar leaves. Chemosphere 146:1–7. https://doi.org/10.1016/j.chemosphere.2015.11.103
ATSDR (2019) ATSDR’s Substance Priority List. Web https://www.atsdr.cdc.gov/spl/index.html#2019spl
Beckers F, Rinklebe J (2017) Cycling of mercury in the environment: sources, fate, and human health implications: A review. Crit Rev Environ Sci Technol 47:693–794. https://doi.org/10.1080/10643389.2017.1326277
Bhat SA, Bashir O, Ul Haq SA, Amin T, Rafiq A, Ali M, Américo-Pinheiro JHP, Sher F (2022) Phytoremediation of heavy metals in soil and water: an eco-friendly, sustainable and multidisciplinary approach. Chemosphere 303:134788. https://doi.org/10.1016/j.chemosphere.2022.134788
Box GEP, Cox DR (1964) An analysis of transformations. J Royal Stat Soc 26:211–252. https://www.jstor.org/stable/2984418
Burleigh SH, Kristensen BK, Bechmann IE (2003) A plasma membrane zinc transporter from Medicago truncatula is up-regulated in roots by Zn fertilization, yet down-regulated by arbuscular mycorrhizal colonization. Plant Mol Biol 52:1077–1088. https://doi.org/10.1023/A:1025479701246
Calgaroto NS, Cargnelutti D, Rossato LV, Farias JG, Nunes ST, Tabaldi LA, Antes FG, Flores EMM, Schetinger MRC, Nicoloso FT (2011) Zinc alleviates mercury-induced oxidative stress in Pfaffia glomerata (Spreng.) Pedersen. Biometals 24:959–971. https://doi.org/10.1007/s10534-011-9457-y
Chamba I, Rosado D, Kalinhoff C, Thangaswamy S, Sánchez-Rodríguez A, Gazquez MJ (2017) Erato polymnioides – a novel Hg hyperaccumulator plant in ecuadorian rainforest acid soils with potential of microbe-associated phytoremediation. Chemosphere 188:633–641. https://doi.org/10.1016/j.chemosphere.2017.08.160
Debeljak M, van Elteren JT, Špruk A, Izmer A, Vanhaecke F, Vogel-Mikuš K (2018) The role of arbuscular mycorrhiza in mercury and mineral nutrient uptake in maize. Chemosphere 212:1076–1084. https://doi.org/10.1016/j.chemosphere.2018.08.147
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: sources, pathways, and effects. Environ Sci Technol 47:4967–4983. https://doi.org/10.1021/es305071v
EPA (2018) Global sourcs of mercury. https://www.epa.gov
Esteban E, Moreno E, Peñalosa J, Cabrero JI, Millán R, Zornoza P (2008) Short and long-term uptake of Hg in white lupin plants: kinetics and stress indicators. Environ Exp Bot 62:316–322. https://doi.org/10.1016/j.envexpbot.2007.10.006
Fariduddin Q, Saleem M, Khan T A, Hayat S (2022) Zinc as a versatile element in plants: an overview on its uptake, translocation, assimilatory roles, deficiency and toxicity symptoms. Microbial Biofertilizers and Micronutrient Availability: The Role of Zinc in Agriculture and Human Health. Springer International Publishing, Cham, 137–158. https://doi.org/10.1007/978-3-030-76609-2_7
Ferrol N, Tamayo E, Vargas P (2016) The heavy metal paradox in arbuscular mycorrhizas: from mechanisms to biotechnological applications. J Exp Bot 67:6253–6265. https://doi.org/10.1093/jxb/erw403
Fiqri A, Utomo WH, Handayanto E (2016) Effect of arbuscular mycorrhizal fungi on the potential of three wild plant species for phytoextraction of mercury from small-scale gold mine tailings. J Degrad Mining Lands Manag 3:551–558. https://doi.org/10.15243/jdmlm.2016.033.551
Garcia J, Barker DG, Journet E-P (2006) Seed storage and germination. The Medicago truncatula handbook. Samuel Roberts Noble Foundation, Ardmore, USA, 1–9
Garg N, Singh S (2018) Arbuscular Mycorrhiza Rhizophagus irregularis and silicon modulate growth, proline biosynthesis and yield in Cajanus cajan L. Millsp. (pigeonpea) genotypes under cadmium and zinc stress. J Plant Growth Regul 37:46–63. https://doi.org/10.1007/s00344-017-9708-4
Gworek B, Dmuchowski W, Baczewska-Dąbrowska AH (2020) Mercury in the terrestrial environment: a review. Environ Sci Eur 32:128. https://doi.org/10.1186/s12302-020-00401-x
Huang X, Ho S-H, Zhu S, Ma F, Wu J, Yang J, Wang L (2017) Adaptive response of arbuscular mycorrhizal symbiosis to accumulation of elements and translocation in Phragmites australis affected by cadmium stress. J Environ Manage 197:448–455. https://doi.org/10.1016/j.jenvman.2017.04.014
Jensen WB (2003) The place of zinc, cadmium, and mercury in the periodic table. J Chem Educ 80:952. https://doi.org/10.1021/ed080p952
Kaur H, Garg N (2018) Recent perspectives on cross talk between cadmium, zinc, and arbuscular mycorrhizal fungi in plants. J Plant Growth Regul 37:680–693. https://doi.org/10.1007/s00344-017-9750-2
Kodre A, Arčon I, Debeljak M, Potisek M, Likar M, Vogel-Mikuš K (2017) Arbuscular mycorrhizal fungi alter Hg root uptake and ligand environment as studied by X-ray absorption fine structure. Environ Exp Bot 133:12–23. https://doi.org/10.1016/j.envexpbot.2016.09.006
Kumar B, Smita K, Cumbal Flores L (2017) Plant mediated detoxification of mercury and lead. Arab J Chem 10:S2335–S2342. https://doi.org/10.1016/j.arabjc.2013.08.010
Kumari S, Amit JR, Mishra N, Singh DK (2020) Recent developments in environmental mercury bioremediation and its toxicity: a review. Environmental Nanotechnology, Monitoring & Management 13:100283. https://doi.org/10.1016/j.enmm.2020.100283
Lehmann A, Veresoglou SD, Leifheit EF, Rillig MC (2014) Arbuscular mycorrhizal influence on zinc nutrition in crop plants – a meta-analysis. Soil Biol Biochem 69:123–131. https://doi.org/10.1016/j.soilbio.2013.11.001
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci U S A 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Ma Y, Wang G, Wang Y, Dai W, Luan Y (2021) Mercury uptake and transport by plants in aquatic environments: a meta-analysis. App Sci 11. https://doi.org/10.3390/app11198829
Manoj SR, Karthik C, Kadirvelu K, Arulselvi PI, Shanmugasundaram T, Bruno B, Rajkumar M (2020) Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: a review. J Environ Manage 254:109779. https://doi.org/10.1016/j.jenvman.2019.109779
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43. https://doi.org/10.1046/j.1469-8137.2002.00363.x
Mercy L, Lucic-Mercy E, Nogales A, Poghosyan A, Schneider C, Arnholdt-Schmitt B (2017) A functional approach towards understanding the role of the mitochondrial respiratory chain in an endomycorrhizal symbiosis. Front Plan Sci 8.https://doi.org/10.3389/fpls.2017.00417
Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, Vandesompele J (2009) A novel and universal method for microRNA RT-qPCR data normalization. Genome Biology 10:R64. https://doi.org/10.1186/gb-2009-10-6-r64
Molina A S, Lugo M A, Pérez Chaca M V, Vargas-Gil S, Zirulnik F, Leporati J, Ferrol N, Azcón-Aguilar C (2020) Effect of arbuscular mycorrhizal colonization on cadmium-mediated oxidative stress in glycine max (L.) Merr Plants 9:108. https://doi.org/10.1007/s00572-021-01056-z
Motaharpoor Z, Taheri H, Nadian H (2019) Rhizophagus irregularis modulates cadmium uptake, metal transporter, and chelator gene expression in Medicago sativa. Mycorrhiza 29:389–395. https://doi.org/10.1007/s00572-019-00900-7
Ruytinx J, Kafle A, Usman M, Coninx L, Zimmermann SD, Garcia K (2020) Micronutrient transport in mycorrhizal symbiosis; zinc steals the show. Fungal Biol Rev 34:1–9. https://doi.org/10.1016/j.fbr.2019.09.001
Saboor A, Ali MA, Danish S, Ahmed N, Fahad S, Datta R, Ansari MJ, Nasif O, Glick BR (2021) Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci Rep 11:18468. https://doi.org/10.1038/s41598-021-97742-1
Salazar MJ, Menoyo E, Faggioli V, Geml J, Cabello M, Rodriguez JH, Marro N, Pardo A, Pignata ML, Becerra AG (2018) Pb accumulation in spores of arbuscular mycorrhizal fungi. Sci Total Environ 643:238–246. https://doi.org/10.1016/j.scitotenv.2018.06.199
Schaefer JK, Szczuka A, Morel FMM (2014) Effect of divalent metals on Hg(II) uptake and methylation by bacteria. Environ Sci Technol 48:3007–3013. https://doi.org/10.1021/es405215v
Senovilla M, Abreu I, Escudero V, Cano C, Bago A, Imperial J, González-Guerrero M (2020) MtCOPT2 is a Cu + transporter specifically expressed in Medicago truncatula mycorrhizal roots. Mycorrhiza 30:781–788. https://doi.org/10.1007/s00572-020-00987-3
Shabani L, Sabzalian MR, Mostafavi pour S (2016) Arbuscular mycorrhiza affects nickel translocation and expression of ABC transporter and metallothionein genes in Festuca arundinacea. Mycorrhiza 26:67–76. https://doi.org/10.1007/s00572-015-0647-2
Singh G, Pankaj U, Chand S, Verma RK (2019) Arbuscular mycorrhizal fungi-assisted phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil and Sediment Contamination: an International Journal 28:729–746. https://doi.org/10.1080/15320383.2019.1657381
Smith S E, Read D (2008) Mycorrhizal symbiosis. Mycorrhizal Symbiosis (Third Edition). Academic Press, London, 1–9. https://doi.org/10.1016/B978-012370526-6.50002-7
Stephens BW, Cook DR, Grusak MA (2011) Characterization of zinc transport by divalent metal transporters of the ZIP family from the model legume Medicago truncatula. Biometals 24:51–58. https://doi.org/10.1007/s10534-010-9373-6
Szczuka A, Morel FMM, Schaefer JK (2015) Effect of thiols, zinc, and redox conditions on Hg uptake in Shewanella oneidensis. Environ Sci Technol 49:7432–7438. https://doi.org/10.1021/acs.est.5b00676
Trouvelot A, Kough J L, Gianinazzi-Pearson V (1986) Mesure du taux de mycorhization VA d’un système radiculaire. Recherche de méthodes d'estimation ayant une signification fonctionnelle. V. Gianinazzi-Pearson, S. Gianinazzi (Eds.). Physiol Gene Asp Mycorr INRA Press Paris 217–221
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(research0034):0031. https://doi.org/10.1186/gb-2002-3-7-research0034
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. https://doi.org/10.1007/s00572-005-0033-6
Wang J, Ma LQ, Letcher R, Bradford S A, Feng X, Rinklebe J (2022) Biogeochemical cycle of mercury and controlling technologies: publications in critical reviews in environmental science & technology in the period of 2017–2021. Crit Rev Environ Sci Technol 1-6. https://doi.org/10.1080/10643389.2022.2071210
Wang L, Huang X, Ma F, Ho S-H, Wu J, Zhu S (2017) Role of Rhizophagus irregularis in alleviating cadmium toxicity via improving the growth, micro- and macroelements uptake in Phragmites australis. Environ Sci Pollut Res 24:3593–3607. https://doi.org/10.1007/s11356-016-7984-3
Wang X, Tam NF-Y, Fu S, Ametkhan A, Ouyang Y, Ye Z (2014) Selenium addition alters mercury uptake, bioavailability in the rhizosphere and root anatomy of rice (Oryza sativa). Ann Bot 114:271–278. https://doi.org/10.1093/aob/mcu117
Watts-Williams SJ, Tyerman SD, Cavagnaro TR (2017) The dual benefit of arbuscular mycorrhizal fungi under soil zinc deficiency and toxicity: linking plant physiology and gene expression. Plant Soil 420:375–388. https://doi.org/10.1007/s11104-017-3409-4
Weissenhorn I, Leyval C, Belgy G, Berthelin J (1995) Arbuscular mycorrhizal contribution to heavy metal uptake by maize (Zea mays L.) in pot culture with contaminated soil. Mycorrhiza 5:245–251. https://doi.org/10.1007/BF00204957
Yon YR, Pérez LA, Carmona AM, Pérez YM, García LRM, Suárez KF, Echevarría AM (2015) Alternative stainning technique to determine mycorrhizal colonization. Cultivos Tropicales 36:18–21. https://doi.org/10.13140/RG.2.2.10232.65287
Yu Y, Zhang S, Huang H (2010) Behavior of mercury in a soil–plant system as affected by inoculation with the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 20:407–414. https://doi.org/10.1007/s00572-009-0296-4
Acknowledgements
The authors are grateful to Australian Pastures Genebank and Inoq GmbH for providing us seeds (Medicago truncatula) and AMF inoculum (Rhizophagus irregularis), respectively. We thank Evans Were for technical help in the laboratory. We further thank Prof. Dr. Hans-Peter Piepho for his help with statistical analysis. We also appreciate the highly valuable comments of the editor and two anonymous reviewers, which helped to improve our manuscript substantially.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Bundesministerium für Bildung und Forschung (BMBF, Germany) under grant no. 01LZ1709A-B and by the China Scholarship Council (CSC) with a stipendium (201706350043).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Experiments and data collection were performed by Yaqin Guo and Nadine Sommer. Data analysis and preparation of the first draft were done by Yaqin Guo. All authors commented and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guo, Y., Sommer, N., Martin, K. et al. Rhizophagus irregularis improves Hg tolerance of Medicago truncatula by upregulating the Zn transporter genes ZIP2 and ZIP6. Mycorrhiza 33, 23–32 (2023). https://doi.org/10.1007/s00572-022-01100-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-022-01100-6