Abstract
Purpose
The healthcare workers are at the greatest risk of being exposed to viral infection during airway management of a patient with coronavirus disease 2019 (COVID-19). An air extractor which suctions air around the patient's face would reduce the spread of viral aerosols during coughing, but no study has confirmed this. We assessed whether or not an air extractor reduces the amount of aerosols spreading toward the operator's face, during coughing of simulated patients.
Methods
After obtained approval of the study by a research ethics committee and written informed consent from 20 volunteers (and additional 20 volunteers), we asked each volunteer to lie supine on a table in a positive-pressure management operating room. As a cross-over design, we used an airborne particle counter (Handheld 3016, SGY company, Tokyo) to measure the aerosols approximately 30 cm above the participant's mouth, while the volunteer was coughing, with and without the use of an air extractor Free-100 M (Forest-one, Funabashi), facing the participant's mouth. In another 20 volunteers, the aerosols were measured, while each volunteer was lying supine, without coughing, and without the use of the air extractor.
Results
The aerosol count during coughing was significantly lower when the air extractor was used [median: 55 (interquartile range: 15–128)] than when it was not used [73 (44–201)] [p = 0.001, difference: 19 (95%CI: 4–70)].
Conclusions
The Free-100 M air extractor would reduce, but do not remove all, aerosols produced by coughing of a patient, and thus may reduce the risk of infection of COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome-corona virus-2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), is highly contagious, and is mainly transmitted through droplets and aerosols emitted from a patient with COVID-19, so that the healthcare workers are at the greatest risk of being exposed to viral infection during airway management [1,2,3,4].
Several methods have been proposed to protect healthcare workers from infection during airway management [1,2,3]. Standard personal protective equipment (PPE), such as P2/N95 masks, goggles, gloves, face-shields, and gowns, is recommended to wear during airway management, but it may not fully prevent viral infection. An "aerosol containment device", which covers the patient's head or the upper body, has been proposed to prevent spread of droplets and aerosols during airway management, but studies have shown that its use may not reduce, but may even increase, the risk of healthcare workers being exposed to a higher concentration of viral aerosols [3]. To prevent the problem of viral aerosols to escape out of an aerosol containment device, some have produced a negative air-flow environment by applying a suction mechanism [2]. One major problem with an aerosol containment device (with or without applying suction mechanism) is that airway management becomes more difficult [3, 5]. In addition, the spread of aerosols is marked when the patient is coughing, whereas it is minimal after rapid-sequence induction of anesthesia and neuromuscular blockade [6], so that the healthcare workers are at the greatest risk of being exposed to viral aerosols before or during placement of an aerosol containment device.
We considered that an alternative method would be to place an air extractor to suction air around the patient's face (without placing an aerosol containment device). As a preliminary study, we carried out a simulation study [4], and have shown that an air extractor could successfully remove simulated viral aerosols, which were produced using a small-volume nebulizer placed in the mannequin’s mouth. Nevertheless, the velocity and the area of spreading droplets and aerosols during real coughing may be different from those of simulated aerosols, and thus the efficacy is not known of an air extractor in minimizing aerosol spread during coughing.
The aim of the current study was to assess whether or not an air extractor reduces the amount of aerosols spreading toward the operator's face, during coughing of simulated patients.
Methods
We considered that it would be ethically not acceptable to study patients, as the efficacy of an air extractor has not been validated in humans, and thus we decided to carry out a study in 20 volunteers who had received vaccinations, whose body temperature had been within normal limits (< 37.5 degrees Celsius) for the last 4 weeks, and had not been close contacts with patients infected with SARS-CoV-2.
Research ethics committee approved the study (approval number: 21002), and we obtained written informed consent from all the volunteered participants. Although this was an observational study, we have registered this study with JRCT (Japan Registry of Clinical Trials: jRCT1032210212).
As a prospective crossover design, we asked each participant to lie supine on an operating table (with the height of approximately 1.0 m) which was placed in the center of an operating room with clean laminar airflow entering through the ceiling and exiting through wall vents, keeping the room at positive pressure.
We used a handheld airborne particle counter (Airborne particle counter Handheld 3016, SGY company, Tokyo, Japan), which can simultaneously count particles of 6 different sizes (0.3, 0.5, 1.0, 2.5, 5.0 and 10.0 μm), and displays cumulative and differential data for particle counts. The measurement efficiency of the counter is 50% at 0.3 μm and 100% at 0.45 μm or more (ISO 21501–4 compliant).
The particle counter was fixed to an intravenous stand. The monitor port was set approximately 30 cm above the participant's mouth. This distance was chosen, because an operator's face would be approximately 30 cm above the patient's mouth, when the operator performs airway management (manual ventilation using a facemask, insertion of a supraglottic airway, or tracheal intubation).
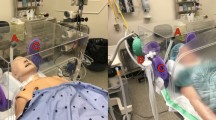
To suction air around the patient's nose and the mouth, we used the Free-100 M (Forest-one, Funabashi, Japan) [4], which consists of a suction port, a length-variable swing arm, and directed high flow suction (with a 12-phase power vacuum), with ultra-low penetration air (ULPA) filter that removes 99.99% of 0.15 µm airborne pathogens (Fig. 1). The level of suction volume can be adjusted from 1 to 12, with a suction speed of 800 l.min−1 at the level 1 setting, and of 3,650 min−1 at the level 12 setting. For this study, we set the suction level of 12.
The air extractor was placed by the operating table, and its suction port was placed approximately 20 cm above of the participant's chest, facing the participant's mouth. The amount of aerosols during coughing were measured, with and without the use of the air extractor, while the participant was coughing three times (of separate timings) in 30 s. The air extractor was used on three sessions of coughing, whereas the air extractor was not used on the other three sessions of coughing. Each experiment was conducted twice per person, and mean values were used for analysis. Timings of the use and non-use of the air extractor were made in a random order. The random order was made using a block randomization (in block of 6). For each measurement, the amount of aerosols were counted for 30 s. We counted the total number of aerosols of 0.3, 0.5, 1.0, 2.5 and 5.0 μm for each session. We also measured the amount of aerosols in the operating room, with the same setting, but without a volunteer on the operating table, and without running the air extractor.
The primary outcome measure was aerosol counts, and the main hypothesis was that aerosol counts would be lower when the air extractor was used than when it was not used. Wilcoxon signed rank sum test was used to compare the aerosol counts with and without the air extractor, as the data were not normally distributed. P < 0.05 was judged significant. The 95% confidence intervals for the median difference in the aerosol counts were also calculated to estimate the difference.
Power analysis was carried out for the primary outcome measure (the aerosol counts during coughing, with and without the use of the air extractor). The null hypothesis for this was that there was no higher or lower aerosol counts between the two circumstances, and thus the proportion of an event (either higher or lower) in the population (π) was 0.5. Cohen [7] defined the following effect size (g = π–0.5) conventions: small (0.05), medium (0.15), and large (0.25). We considered that the difference with and without the use of air extractor would be clinically meaningful, when the effect size was larger than the Cohen’s “large” (0.25), and thus we defined the effect size of 0.3 would be clinically meaningful. Twenty patients would be required to detect this size of the difference, with a power of 0.8, and P = 0.05.
Responding to a reviewer’s comments to the initial draft, we carried out an additional study. Research ethics committee approved this additional study (approval number: 22088), and we obtained written informed consent from all the volunteer participants. Because this was an additional study, 20 volunteers in this part of the study were different from those for the main study.
In this additional study, we asked each participant to lie supine on an operating table with the same settings for the main part of the study. The amount of aerosols was measured, while each volunteer was asked not to cough, and without running the air extractor. Mann–Whitney U test was used to compare the aerosol counts with and without the presence of a volunteer on an operating table (as the data were not paired). P < 0.05 was judged significant, as this was regarded as a subsidiary information.
Results
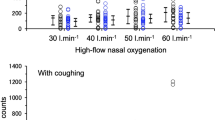
The amount of aerosols in the operating room, without a volunteer on the operating table, and without running the air extractor were 0 in 17 of 20 measurements, and 1 to 6 counts in the remaining 3 measurements, indicating that the amounts of aerosols in the operating room are approximately 0 (Fig. 2). An additional study has indicted that with a volunteer on the operating table, not coughing, and without running the air extractor, the amount of aerosols varied from 0 to 72, with the median of 3.5 (Fig. 2). The amount of aerosol measured was significantly higher when a volunteer was present than when a volunteer was absent on the table (P = 0.0043).
Aerosol count (30 cm above the participant's mouth) during coughing, with and without the use of an air extractor (Free-100 M, Forest-one, Funabashi, Japan), and while a volunteer was or was not lying on an operating table (without suctioning of air) (scatter plots with the medians and interquartile ranges). A dotted vertical line is drawn, as the data for “volunteer, not coughing, without suction” were taken from different volunteers from those in the main part of the study
The aerosol count during coughing was significantly lower when the suction system was used [median: 55 (interquartile range: 15–128)] than when it was not used [73 (44–201)] [p = 0.001, difference: 19 (4–70)] (Fig. 2).
Discussion
We have confirmed that Free-100 M air extractor significantly decreased the amount of aerosols spread by coughing of simulated patients toward the operator's face, but a considerable amount of aerosols was sometimes detected even when the air extractor was being used.
In a previous simulation study, we have visually confirmed that the air extractor could remove all the simulated viral aerosols, which were produced using a small-volume nebulizer placed in the manikin’s mouth [4]. Another study has also shown that an air extractor was effective in removing aerosols during simulated continuous breathing and a manikin-simulated cough [8]. Therefore, these simulation studies have indicated that an air extractor can prevent spread of viral aerosols emitted during coughing.
Although we did not measure the volume of air emitted from each participant, the volume of air emitted by coughing should be less than the forced expiratory volume, and has been reported to be 0.5 l to 4.8 l, with the peak flow rate of up to approximately 930 l.min−1 (or 16 l.sec−1)[9]. The Free-100 M air extractor can suction up to 3,650 l.min−1 (60 l.sec−1) of air, so that suction volumes would be far much greater than the maximum volume of air emitted during coughing. Since the Free-100 M air extractor can suction 1,200 l.min−1 (20 l.sec−1) of air at the level 2 setting, the Free-100 M air extractor, in theory, can suction expired air during coughing by setting the suction level of 2 or greater.
Despite the fact that these simulation studies and theoretical calculations indicate that the air extractor can effectively prevent spread of viral aerosols emitted by coughing, this study has shown that the Free-100 M air extractor reduces, but cannot remove all, the aerosols emitted by coughing. The reason is not clear for the considerably high aerosols were detected around the operator’s face during real coughing, while an air extractor was kept running. Nevertheless, one possible reason is that aerosols produced by coughing spread radially with a high velocity, so that a small suction port of the air extractor placed above a participant’s chest could not effectively suction the aerosols spread around the suction port. In our study, the suction port of the air extractor was placed approximately 20 cm above of the participant's chest, facing the participant's mouth. The efficacy of the air extractor may well have been affected by the location and the direction of the suction port, so that this positioning might not have been the optimal.
Limitations of the study include that we did not study how effectively the use of an air extractor reduces the incidence of infection of the operator (true outcome measure). Nevertheless, it would be reasonable to deduce that the use of air extractor would reduce the spread of aerosols around an operator’s face, and that its use would reduce, but may not reliably prevent, the risk of infection of operator.
Another limitation is that it is not clear whether or not all the amount of the aerosols detected by the counter were emitted from the participant’s airway during coughing. In a preliminary study, we noticed that the aerosol count in an “ordinary” room (even without anyone there) could be as high as 10,000, and this count did not change even when the air extractor was turned on. This indicates that in an “ordinary” room contains relatively high density of aerosols, and even if an air extractor effectively removes the air, the surrounding air (which would contain the same density of aerosols) would fill the space. Therefore, it would be technically difficult to determine how effectively the air extractor can remove the aerosols emitted from a patient.
To minimize this difficulty, we chose to study in an operating room with positive-pressure management, where the density of aerosols in the air is much lower than the air in an “ordinary” room, and in fact, the count of aerosols in the operating room, where no volunteer was on the operating table was 0 or almost 0. An additional study has indicated that the amount of aerosols when a volunteer was lying on a table but not coughing ranged from 0 to 72, and although it was not a direct comparison, the amount was significantly higher than the amount when a volunteer was not lying on the table, but significantly lower than the amount when a volunteer was coughing. Therefore, aerosols detected during coughing would mainly from the person coughing, and that the air extractor could not remove aerosols produced by coughing.
Clinical implications of this study include that, because the Free-100 M air extractor can reduce, but may not remove all, the aerosols emitted by coughing, the use of air extractor cannot make the standard PPE unnecessary, when the patient is known to be, or suspected to be, infected. Nevertheless, because PPE may not fully prevent viral infection, additional use of an air extractor to wearing PPE would reduce the risk further. It is known that viral aerosols may be coming out even from asymptomatic patients with negative test results for COVID-19 [10]. Therefore, an air extractor may be routinely used, to minimize a possible risk of infection to healthcare workers who are working by the patient. The Free-100 M is a transportable compact system, so that it is easy to transport it to the patient's side, and to start suctioning, even when an emergency airway management is required.
In conclusion, the Free-100 M air extractor would reduce exposure of healthcare providers to infectious aerosols emitted by coughing, but a considerable amount of aerosols may still be exposed to healthcare providers.
Data availability
Data are available on request.
References
Yamakage M. Anesthesia in the times of COVID-19. J Anesth. 2021;35:325–7.
Hirose K, Uchida K, Umezu S. Airtight, flexible, disposable barrier for extubation. J Anesth. 2020;34:798–9.
Saito T, Asai T. Aerosol containment device for airway management of patients with COVID-19: narrative review. J Anesth. 2021;35:384–9.
Saito T, Fujishiro A, Asai T. Aerosol extractor for airway management of COVID-19 patients. J Anesth. 2021;35:323.
Saito T, Taguchi A, Asai T. Videolaryngoscopy for tracheal intubation in patients with COVID-19. Br J Anaesth. 2020;125:e284–6.
Simpson JP, Wong DN, Verco L, Carter R, Dzidowski M, Chan PY. Measurement of airborne particle exposure during simulated tracheal intubation using various proposed aerosol containment devices during the COVID-19 pandemic. Anaesthesia. 2020;75:1587–95.
Cohen J. The test that a proportion is 50 and the sign test In Statistical power analysis for the behavioral sciences. 2nd ed. New York: Psychology press; 1988. https://doi.org/10.1016/B978-0-12-179060-8.50010-4.
Matava C, Collard V, Siegel J, Denning S, Li T, Du B, Fiadjoe J, Fiset P, Engelhardt T. Use of a high-flow extractor to reduce aerosol exposure in tracheal intubation. Br J Anaesth. 2020;125(4):363–6.
Mahajan RP, Singh P, Murphy GE, Aitkenhead AR. Relationship between expired lung volume, peak flow rate and peak velocity time during a voluntary cough manoeuvre. Br J Anaesth. 1994;72:298–301.
Asai T. Covid-19: accurate interpretation of diagnostic tests-a statistical point of view. J Anesth. 2021;35:328–32.
Funding
AF received Research incentive grant from Dokkyo Medical University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, data collection and analysis. AF, TS and TA drafted the first manuscript, and all revising it critically for important intellectual content. All agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Fujishiro, A., Asai, T., Saito, T. et al. Efficacy of an aerosol suction device Free-100 M in removing aerosols produced by coughing to minimize COVID-19 infection. J Anesth 37, 196–200 (2023). https://doi.org/10.1007/s00540-022-03144-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-022-03144-6