Abstract
Background
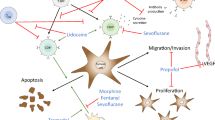
The issue whether anaesthesia has an impact on the prognosis of carcinoma has been widely discussed and remains debated. Ropivacaine has been widely used in perioperative period as a long acting local anesthetic. An early event during recurrence or metastasis of carcinoma is the adhesion of circulating tumour cells (CTCs) to endothelial cells (ECs) through binding adhesion molecules that are up-regulated on inflamed endothelium during the perioperative period or other periods. This study was to explore the impact of ropivacaine on the adhesion of tumour cells, providing evidences of its influence on the prognosis of carcinoma.
Materials and methods
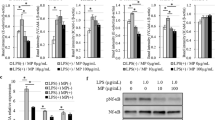
Human umbilical vein endothelial cells (HUVECs) were pre-treated with ropivacaine (10–7–10–5 M; 30 min) prior to treatment with tumour necrosis factor alpha (TNFα) (10 ng ml−1; 1, 4 and 8 h). Intercellular adhesion molecule-1 (ICAM-1), endothelial-selectin (CD62E) and vascular cell adhesion molecule-1 (VCAM-1) mRNA levels were detected via quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). To clarify the underlying action mechanism, p65, p-p65, IκBα, p-IκBα, IKKα/β and p-IKKα/β protein levels were evaluated via western blotting. Cell viability and tumour cell adhesion assays were also assessed.
Results
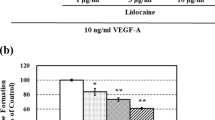
The clinically usage concentration of ropivacaine (10–6 M) produced a significant decrease in CD62E expression compared with that produced by TNFα only (p < 0.001). Moreover, adhesion assays showed that ropivacaine effectively inhibited the adhesion of hepatoma cells (p < 0.01), human colon cancer cells (p < 0.01) and human leukemic monocyte (p < 0.01). Western blot results showed that pre-treatment with ropivacaine inhibited the phosphorylation of p65 (p < 0.05), IκBα (p < 0.001) and IKKα/β (p < 0.01).
Conclusions
Ropivacaine decreased the adhesion of tumour cells. Ropivacaine modulated CD62E expression by inhibiting the activation of NF-κB. These results might provide new insight into the issue whether anaesthesia has an impact on the prognosis of carcinoma.







Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386386.
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660.
Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–7.
Lin L, Liu C, Tan H, Ouyang H, Zhang Y, Zeng W. Anaesthetic technique may affect prognosis for ovarian serous adenocarcinoma: a retrospective analysis. Br J Anaesth. 2011;106(6):814–22.
Gupta A, Björnsson A, Fredriksson M, Hallböök O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107(2):164–70.
Peach G, Kim C, Zacharakis E, Purkayastha S, Ziprin P. Prognostic significance of circulating tumour cells following surgical resection of colorectal cancers: a systematic review. Br J Cancer. 2010;102(9):1327–34.
Eschwege P, Dumas F, Blanchet P, Le Maire V, Benoit G, Jardin A, Lacour B, Loric S. Haematogenous dissemination of prostatic epithelial cells during radical prostatectomy. Lancet (London, England). 1995;346(8989):1528–30.
Neeman E, Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30:S32–S40.
Lin E, Calvano SE, Lowry SF. Inflammatory cytokines and cell response in surgery. Surgery. 2000;127(2):117–26.
Yamaguchi K, Takagi Y, Aoki S, Futamura M, Saji S. Significant detection of circulating cancer cells in the blood by reverse transcriptase–polymerase chain reaction during colorectal cancer resection. Ann Surg. 2000;232(1):58.
Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138(5):1714–26 (e1713).
Reymond N, d'Agua BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70.
St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci (Landmark Ed). 2011;16:3233–51.
Läubli H, Borsig L. Selectins promote tumor metastasis. Semin Cancer Biol. 2010;20(3):169–77.
Yoshimoto K, Tajima H, Ohta T, Okamoto K, Sakai S, Kinoshita J, Furukawa H, Makino I, Hayashi H, Nakamura K, Oyama K, Inokuchi M, Nakagawara H, Itoh H, Fujita H, Takamura H, Ninomiya I, Kitagawa H, Fushida S, Fujimura T, Wakayama T, Iseki S, Shimizu K. Increased E-selectin in hepatic ischemia-reperfusion injury mediates liver metastasis of pancreatic cancer. Oncol Rep. 2012;28(3):791–6.
Gebauer F, Wicklein D, Stübke K, Nehmann N, Schmidt A, Salamon J, Peldschus K, Nentwich MF, Adam G, Tolstonog G, Bockhorn M, Izbicki JR, Wagener C, Schumacher U. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp−−/rag2−− mice. Gut. 2013;62(5):741–50.
Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, Jain RK. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci USA. 2011;108(9):3725–30.
Hill CAS, Baharo-Hassan D, Farooqui M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS ONE. 2011;6(1):e16281.
Zhang B-H, Chen H, Yao X-P, Cong W-M, Wu M-C. E-selectin and its ligand-sLeX in the metastasis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1(1):80–2.
Walz G, Aruffo A, Kolanus W, Bevilacqua M, Seed B. Recognition by ELAM-1 of the sialyl-Lex determinant on myeloid and tumor cells. Science. 1990;250(4984):1132–5.
Montgomery KF, Osborn L, Hession C, Tizard R, Goff D, Vassallo C, Tarr PI, Bomsztyk K, Lobb R, Harlan JM. Activation of endothelial-leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Proc Natl Acad Sci USA. 1991;88(15):6523–7.
Takada K, Nakane T, Masuda K, Ishii H. Ursolic acid and oleanolic acid, members of pentacyclic triterpenoid acids, suppress TNF-α-induced E-selectin expression by cultured umbilical vein endothelial cells. Phytomedicine. 2010;17(14):1114–9.
Hayden MS, Ghosh S. Shared principles in NF-κB signaling. Cell. 2008;132(3):344–62.
Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–7.
Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–15.
Piegeler T, Votta-Velis EG, Bakhshi FR, Mao M, Carnegie G, Bonini MG, Schwartz DE, Borgeat A, Beck-Schimmer B, Minshall RD. Endothelial barrier protection by local anesthetics: ropivacaine and lidocaine block tumor necrosis factor-alpha-induced endothelial cell Src activation. Anesthesiology. 2014;120(6):1414–28.
Piegeler T, Dull RO, Hu G, Castellon M, Chignalia AZ, Koshy RG, Votta-Velis EG, Borgeat A, Schwartz DE, Beck-Schimmer B, Minshall RD. Ropivacaine attenuates endotoxin plus hyperinflation-mediated acute lung injury via inhibition of early-onset Src-dependent signaling. BMC Anesthesiol. 2014;14:57.
Baudin B, Bruneel A, Bosselut N, Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc. 2007;2(3):481–5.
Lu Y, Yu T, Liang H, Wang J, Xie J, Shao J, Gao Y, Yu S, Chen S, Wang L, Jia L. Nitric oxide inhibits hetero-adhesion of cancer cells to endothelial cells: restraining circulating tumor cells from initiating metastatic cascade. Sci Rep. 2014;4:4344.
Perotti L, Cusato M, Ingelmo P, Niebel TL, Somaini M, Riva F, Tinelli C, De Andrés J, Fanelli G, Braschi A, Regazzi M, Allegri M. A comparison of differences between the systemic pharmacokinetics of levobupivacaine and ropivacaine during continuous epidural infusion: a prospective, randomized, multicenter, double-blind controlled trial. Anesth Analg. 2015;121(2):348–56.
Ten Kate M, Hofland LJ, van Grevenstein WMU, van Koetsveld PV, Jeekel J, van Eijck CHJ. Influence of proinflammatory cytokines on the adhesion of human colon carcinoma cells to lung microvascular endothelium. Int J Cancer. 2004;112(6):943–50.
Piegeler T, Votta-Velis EG, Liu G, Place AT, Schwartz DE, Beck-Schimmer B, Minshall RD, Borgeat A. Anti-metastatic potential of amide-linked local anesthetics: inhibition of lung adenocarcinoma cell migration and inflammatory Src signaling independent of sodium channel blockade. Anesthesiology. 2012;117(3):548.
Zhu M, Li M, Zhou Y, Dangelmajer S, Kahlert UD, Xie R, Xi Q, Shahveranov A, Ye D, Lei T. Isoflurane enhances the malignant potential of glioblastoma stem cells by promoting their viability, mobility in vitro and migratory capacity in vivo. Br J Anaesth. 2016;116(6):870–7.
Zervantonakis IK, Hughes-Alford SK, Charest JL, Condeelis JS, Gertler FB, Kamm RD. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc Natl Acad Sci USA. 2012;109(34):13515–20.
Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Blackwell TS, Baron RM, Feinberg MW. MicroRNA-181b regulates NF-κB–mediated vascular inflammation. J Clin Investig. 2012;122(6):1973–90.
Yuan T, Li Z, Li X, Yu G, Wang N, Yang X. Lidocaine attenuates lipopolysaccharide-induced inflammatory responses in microglia. J Surg Res. 2014;192(1):150–62.
Brocco MC, Paulo DNS, Almeida CED, Carraretto AR, Cabral SA, Silveira AC, Gomez RS, Baptista JF. A study of interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) serum levels in rats subjected to fecal peritonitis and treated with intraperitoneal ropivacaine. Acta Cirurgica Brasileira. 2012;27:494–8.
Cusato M, Allegri M, Niebel T, Ingelmo P, Broglia M, Braschi A, Regazzi M. Flip-flop kinetics of ropivacaine during continuous epidural infusion influences its accumulation rate. Eur J Clin Pharmacol. 2011;67(4):399–406.
Wiedemann D, Mühlnickel B, Staroske E, Neumann W, Röse W. Ropivacaine plasma concentrations during 120-hour epidural infusion. Br J Anaesth. 2000;85(6):830–5.
Latzke D, Marhofer P, Kettner SC, Koppatz K, Turnheim K, Lackner E, Sauermann R, Müller M, Zeitlinger M. Pharmacokinetics of the local anesthetic ropivacaine after transversus abdominis plane block in healthy volunteers. Eur J Clin Pharmacol. 2012;68(4):419–25.
Ekatodramis G, Borgeat A, Huledal G, Jeppsson L, Westman L, Sjövall J. Continuous interscalene analgesia with ropivacaine 2 mg/ml after major shoulder surgery. J Am Soc Anesthesiol. 2003;98(1):143–50.
Kaloul I, Guay J, Côté C, Halwagi A, Varin F. Ropivacaine plasma concentrations are similar during continuous lumbar plexus blockade using the anterior three-in-one and the posterior psoas compartment techniques. Can J Anesth. 2004;51(1):52.
Khatib A-M, Kontogiannea M, Fallavollita L, Jamison B, Meterissian S, Brodt P. Rapid induction of cytokine and E-selectin expression in the liver in response to metastatic tumor cells. Cancer Res. 1999;59(6):1356–61.
Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71(4):612–9.
Lucchinetti E, Awad AE, Rahman M, Feng J, Lou PH, Zhang L, Ionescu L, Lemieux H, Thébaud B, Zaugg M. Antiproliferative effects of local anesthetics on mesenchymal stem cells potential implications for tumor spreading and wound healing. Anesthesiology. 2012;116(4):841–56.
Ea C-K, Deng L, Xia Z-P, Pineda G, Chen ZJ. Activation of IKK by TNFα requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22(2):245–57.
Xu M, Skaug B, Zeng W, Chen ZJ. A ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFα and IL-1β. Mol Cell. 2009;36(2):302–14.
Poyet J-L, Srinivasula SM, Lin J-H, Alnemri T, Yamaoka S, Tsichlis PN, Alnemri ES. Activation of the IκB kinases by RIP via IKKγ/NEMO-mediated oligomerization. J Biol Chem. 2000;275(48):37966–77.
Acknowledgements
The authors will thank Dr. Hai Jiang for his great help in pre-experiment execution. This work was supported by the National Natural Science Foundation of China (Grant numbers 30972857, 2010 and 81672405, 2016). The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Su, Z., Huang, P., Ye, X. et al. Ropivacaine via nuclear factor kappa B signalling modulates CD62E expression and diminishes tumour cell arrest. J Anesth 33, 685–693 (2019). https://doi.org/10.1007/s00540-019-02699-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-019-02699-1