Abstract
Background
Neuropeptide S (NPS) is an endogenous neuropeptide controlling anxiolysis, wakefulness, and analgesia. NPS containing neurons exist near to the locus coeruleus (LC) involved in the descending anti-nociceptive system. NPS interacts with central noradrenergic neurons; thus brain noradrenergic signaling may be involved in NPS-induced analgesia. We tested NPS analgesia in noradrenergic neuron-lesioned rats using a selective LC noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4).
Methods
A total 66 male Sprague–Dawley rats weighing 350–450 g were used. Analgesic effects of NPS were evaluated using hot-plate and tail-flick test with or without DSP-4. The animal allocated into 3 groups; hot-plate with NPS alone intracerebroventricular (icv) (0.0, 1.0, 3.3, and 10.0 nmol), tail-flick NPS alone icv (0.0 and 10.0 nmol), and hot-plate with NPS and DSP-4 (0 or 50 mg/kg ip). In hot-plate with NPS and DSP-4 group, noradrenaline content in the cerebral cortex, pons, hypothalamus, were measured.
Results
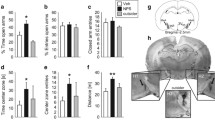
NPS 10 nmol icv prolonged hot plate (%MPE) but not tail flick latency at 30 and 40 min after administration. DSP-4 50 mg/kg decreased noradrenaline content in the all 3 regions. The NA depletion inhibited NPS analgesic effect in the hot plate test but not tail flick test. There was a significant correlation between hot plate latency (percentage of maximum possible effect: %MPE) with NPS 10 nmol and NA content in the cerebral cortex (p = 0.017, r 2 = 0.346) which noradrenergic innervation arisen mainly from the LC. No other regions had the correlation.
Conclusions
NPS analgesia interacts with LC noradrenergic neuronal activity.




Similar content being viewed by others
References
Ruzza C, Calo G, Di Maro S, Pacifico S, Trapella C, Salvadori S, Preti D, Guerrini R. Neuropeptide S receptor ligands: a patent review (2005–2016). Expert Opin Ther Pat. 2017;27:347–62.
Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br J Pharmacol. 2008;154:471–9.
Dine J, Ionescu IA, Stepan J, Yen YC, Holsboer F, Landgraf R, Eder M, Schmidt U. Identification of a role for the ventral hippocampus in neuropeptide S-elicited anxiolysis. PLoS ONE. 2013;8:e60219.
Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, de Lecea L, Civelli O. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–97.
Yang Y, Zhao M, Zhang Y, Shen X, Yuan Y. Correlation of 5-HTT, BDNF and NPSR1 gene polymorphisms with anxiety and depression in asthmatic patients. Int J Mol Med. 2016;38:65–74.
Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology. 2016;63:351–61.
Han RW, Xu HJ, Zhang RS, Wang P, Chang M, Peng YL, Deng KY, Wang R. Neuropeptide S interacts with the basolateral amygdala noradrenergic system in facilitating object recognition memory consolidation. Neurobiol Learn Mem. 2014;107:32–6.
Ruzza C, Pulga A, Rizzi A, Marzola G, Guerrini R, Calo G. Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology. 2012;62:1999–2009.
Beiderbeck DI, Lukas M, Neumann ID. Anti-aggressive effects of neuropeptide S independent of anxiolysis in male rats. Front Behav Neurosci. 2014;8:185.
Ruzza C, Asth L, Guerrini R, Trapella C, Gavioli EC. Neuropeptide S reduces mouse aggressiveness in the resident/intruder test through selective activation of the neuropeptide S receptor. Neuropharmacology. 2015;97:1–6.
Ahnaou A, Drinkenburg WH. Neuropeptide-S evoked arousal with electroencephalogram slow-wave compensatory drive in rats. Neuropsychobiology. 2012;65:195–205.
Oishi M, Kushikata T, Niwa H, Yakoshi C, Ogasawara C, Calo G, Guerrini R, Hirota K. Endogenous neuropeptide S tone influences sleep-wake rhythm in rats. Neurosci Lett. 2014;581:94–7.
Roncace V, Polli FS, Zojicic M, Kohlmeier KA. Neuropeptide S (NPS) is a neuropeptide with cellular actions in arousal and anxiety-related nuclei: functional implications for effects of NPS on wakefulness and mood. Neuropharmacology. 2017;126:292–317.
Kushikata T, Yoshida H, Kudo M, Salvadori S, Calo G, Hirota K. The effects of neuropeptide S on general anesthesia in rats. Anesth Analg. 2011;112:845–9.
Kong XP, Wang C, Xie JF, Zhao P, Dai LR, Shao YF, Lin JS, Hou YP. Neuropeptide S reduces propofol- or ketamine-induced slow wave states through activation of cognate receptors in the rat. Neuropeptides. 2017;63:59–66.
Li W, Gao YH, Chang M, Peng YL, Yao J, Han RW, Wang R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides. 2009;30:234–40.
Peng YL, Zhang JN, Chang M, Li W, Han RW, Wang R. Effects of central neuropeptide S in the mouse formalin test. Peptides. 2010;31:1878–83.
Medina G, Ji G, Gregoire S, Neugebauer V. Nasal application of neuropeptide S inhibits arthritis pain-related behaviors through an action in the amygdala. Mol Pain. 2014;10:32.
Holanda AD, Asth L, Santos AR, de Guerrini R, de PS-RV, Calo G, Andre E, Gavioli EC. Central adenosine A1 and A2A receptors mediate the antinociceptive effects of neuropeptide S in the mouse formalin test. Life Sci. 2015;120:8–12.
Yang F, Peng L, Luo J, Yi H, Hu X. Intra-amygdala microinfusion of neuropeptide S attenuates neuropathic pain and suppresses the response of spinal microglia and astrocytes after spinal nerve ligation in rats. Peptides. 2016;82:26–34.
Clark SD, Duangdao DM, Schulz S, Zhang L, Liu X, Xu YL, Reinscheid RK. Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J Comp Neurol. 2011;519:1867–93.
Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol. 2007;500:84–102.
Okamura N, Garau C, Duangdao DM, Clark SD, Jungling K, Pape HC, Reinscheid RK. Neuropeptide S enhances memory during the consolidation phase and interacts with noradrenergic systems in the brain. Neuropsychopharmacology. 2011;36:744–52.
Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7.
Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–94.
Guerrini R, Camarda V, Trapella C, Calo G, Rizzi A, Ruzza C, Fiorini S, Marzola E, Reinscheid RK, Regoli D, Salvadori S. Synthesis and biological activity of human neuropeptide S analogues modified in position 5: identification of potent and pure neuropeptide S receptor antagonists. J Med Chem. 2009;52:524–9.
Kushikata T, Kubota T, Fang J, Krueger JM. Glial cell line-derived neurotrophic factor promotes sleep in rats and rabbits. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1001–6.
Zaczek R, Fritschy JM, Culp S, De Souza EB, Grzanna R. Differential effects of DSP-4 on noradrenaline axons in cerebral cortex and hypothalamus may reflect heterogeneity of noradrenaline uptake sites. Brain Res. 1990;522:308–14.
Mohammad Ahmadi Soleimani S, Azizi H, Mirnajafi-Zadeh J, Semnanian S. Orexin type 1 receptor antagonism in rat locus coeruleus prevents the analgesic effect of intra-LC met-enkephalin microinjection. Pharmacol Biochem Behav. 2015;136:102–6.
Ross SB, Stenfors C. DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action. Neurotox Res. 2015;27:15–30.
Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. Pharmacokinetics of ketamine and xylazine in young and old Sprague–Dawley rats. J Am Assoc Lab Anim Sci. 2013;52:567–70.
Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand Suppl. 1971;367:1–48.
Kudo T, Kushikata T, Kudo M, Kudo T, Hirota K. A central neuropathic pain model by DSP-4 induced lesion of noradrenergic neurons: preliminary report. Neurosci Lett. 2010;481:102–4.
Ossipov MH, Kovelowski CJ, Nichols ML, Hruby VJ, Porreca F. Characterization of supraspinal antinociceptive actions of opioid delta agonists in the rat. Pain. 1995;62:287–93.
Viguier F, Michot B, Hamon M, Bourgoin S. Multiple roles of serotonin in pain control mechanisms—implications of 5-HT(7) and other 5-HT receptor types. Eur J Pharmacol. 2013;716:8–16.
Acknowledgements
This study was supported by grants-in-aid for scientific research (23592242, 24659690, 25462394 and 26462327) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Grant for Hirosaki University Institutional Research.
Author information
Authors and Affiliations
Contributions
KJ performed data collection, data analysis and prepare the manuscript. KJ approved the final manuscript. KJ attests to the integrity of the original data and the analysis reported in this manuscript. TeK conducted of the study, performed data collection, and prepared the manuscript. TeK approved the final manuscript. TeK attests to the integrity of the original data and the analysis reported in this manuscript. TeK is the archival author. TaK helped design the study and performed data collection, data analysis. TaK approved the final manuscript. GC helped design the study and prepare the manuscript. GC approved the final manuscript. RG helped design the study and prepared the synthesis of Neuropeptide S. RG approved the final manuscript. KH performed data analysis and prepared the manuscript. KH approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Kei Jinushi: None. Tetsuya Kushikata: Grants-in-Aid for surveillance of academic research activity in Japan from Japan Society for the Promotion of Science Nos. 23592242, 24659690, 25462394 and 26462327. Takashi Kudo: None. Girolamo Calo: None. Remo Guerrini: None. Kazuyoshi Hirota: Grants-in-Aid for surveillance of academic research activity in Japan from Japan Society for the Promotion of Science No. 24659690.
About this article
Cite this article
Jinushi, K., Kushikata, T., Kudo, T. et al. Central noradrenergic activity affects analgesic effect of Neuropeptide S. J Anesth 32, 48–53 (2018). https://doi.org/10.1007/s00540-017-2427-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2427-y