Abstract
Background
The transcription factor nuclear factor-E2-related factor-2 (Nrf2) is a key regulator for induction of hepatic antioxidative stress systems. We aimed to investigate whether activation of Nrf2 protects against steatohepatitis.
Method
Wild-type mice (WT), Nrf2 gene-null mice (Nrf2-null) and Keap1 gene-knockdown mice (Keap1-kd), which represent the sustained activation of Nrf2, were fed a methionine- and choline-deficient diet (MCDD) for 13 weeks and analyzed.
Results
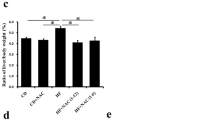
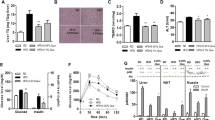
In Keap1-kd fed an MCDD, steatohepatitis did not develop over the observation periods; however, in Nrf2-null fed an MCDD, the pathological state of the steatohepatitis was aggravated in terms of fatty change, inflammation, fibrosis and iron accumulation. In WT mice fed an MCDD, Nrf2 and antioxidative stress genes regulated by Nrf2 were potently activated in the livers, and in Keap1-kd, their basal levels were potently activated. Oxidative stress was significantly increased in the livers of the Nrf2-null and suppressed in the livers of the Keap1-kd compared to that of WT, based on the levels of 4-hydroxy-2-nonenal and malondialdehyde. Iron accumulation was greater in the livers of the Nrf2-null mice compared to those of the WT mice, and it was not observed in Keap1-kd. Further, the iron release from the isolated hepatocyte of Nrf2-null mice was significantly decreased. Sulforaphane, an activator of Nrf2, suppressed the pathological states and oxidative stress in the livers.
Conclusions
Nrf2 has protective roles against nutritional steatohepatitis through inhibition of hepatic iron accumulation and counteraction against oxidative stress-induced liver injury. Nrf2 activation by pharmaceutical intervention could be a new option for the prevention and treatment of steatohepatitis.






Similar content being viewed by others
Abbreviations
- α-Sma:
-
Alpha-smooth muscle actin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- Fpn1:
-
Ferroportin-1
- γ-Gcs:
-
γ-Glutamylcysteine synthetase
- GSH:
-
Glutathione
- Gst:
-
Glutathione S-transferase
- Hamp:
-
Hepcidin gene
- 4-HNE:
-
4-Hydroxy-2-nonenal
- Keap1:
-
Kelch-like Ech-associated protein 1
- MCDD:
-
Methionine- and choline-deficient diet
- MDA:
-
Malondialdehyde
- NASH:
-
Non-alcoholic steatohepatitis
- Nrf2:
-
Nuclear factor-E2-related factor-2
- Nqo1:
-
NAD(P)H: quinone oxidoreductase 1
- ROS:
-
Reactive oxygen species
- SFN:
-
Sulforaphane
- TfR:
-
Transferrin receptor
- Tgf:
-
Transforming growth factor
- WT:
-
Wild type
References
Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98.
Vuppalanchi R, Naga C. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: selected practical issues in their evaluation and management. Hepatology. 2009;49:306–17.
Falch-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26.
Adams LA, Lymp JF, Sauver JS, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population based cohort study. Gastroenterology. 2005;129:113–21.
Malaguarnera L, Madeddu R, Palio E, Arena N, Malaguarnera M. Heme oxygenase-1 levels and oxidative stress-related parameters in non-alcoholic fatty liver disease patients. J Hepatol. 2005;42:585–91.
Sumida Y, Nakashima T, Yoh T, Furutani M, Hirohama A, Kakisaka Y, et al. Serum thioredoxin levels as a predictor of steatohepatitis in patients with nonalcoholic fatty liver disease. J Hepatol. 2003;38:32–8.
Tomita K, Oike Y, Teratani T, Taguchi T, Noguchi M, Suzuki T, et al. Hepatic AdipoR2 signaling plays a protective role against progression of nonalcoholic steatohepatitis in mice. Hepatology. 2008;48:458–73.
Yu J, Chu ES, Wang R, Wang S, Wu CW, Wong VWS, et al. Heme oxygenase-1 protects against steatohepatitis in both cultured hepatocyte and mice. Gastroenterology. 2010;138:694–704.
Leonarduzz G, Scavazza A, Biasi F, Chiarpotto E, Camandola S, Vogel S, et al. The lipid peroxidation end product 4-hydroxy-2,3-nonenal up-regulates transforming growth factor beta expression in the macrophages lineage: a link between oxidative injury and fibrosclerosis. FASEB J. 1997;11:851–7.
Matsuzawa N, Takamura T, Kurita S, Nisu H, Ota T, Ando H, et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 2007;46:1392–403.
Zhang DD, Hannink M. Distinct cysteine in Keap1 are required for Keap1-dependent ubiquitination on Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–51.
Kwak MK, Itoh K, Yamamoto M, Sutter TR, Kensler TW. Role of transcription factor Nrf2 in the induction of hepatic phase 2 and antioxidative enzymes in vivo by the cancer chemoprotective agent, 3H-1, 2-dithiole-3-thione. Mol Med. 2001;7:135–45.
Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–47.
Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, et al. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389:431–6.
Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, et al. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283–94.
Chowdhry S, Nazmy MH, Meakin PJ, Dinkova-Kostova AT, Walsh SV, Tsujita T, et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free Radic Biol Med. 2010;48:357–71.
Zhang YKJ, Yeager RL, Tanaka Y, Klaassen CD. Enhanced expression of Nrf2 in mice attenuated the fatty liver produced by a methionine- and choline-deficient diet. Toxicol Appl Pharmacol. 2010;245:326–34.
Harada N, Kanayama M, Maruyama A, Yoshida A, Tazumi K, Hosoya T, et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch Biochem Biophys. 2011;508:101–9.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3.
George DK, Goldwurm S, Macdonald GA, Cowley LL, Walker NI, Ward PJ, et al. Increased hepatic iron concentration in nonalcoholic steatohepatitis is associated with increased fibrosis. Gastroenterology. 1998;114:311–8.
Shinkai Y, Sumi D, Fukami I, Ishii T, Kumagai Y. Sulforaphane, an activator of Nrf2, suppresses cellular accumulation of arsenic and its cytotoxicity in primary mouse hepatocytes. FEBS Lett. 2006;580:1771–4.
Foy AL, Williams HL, Cortell S, Conrad ME. A modified procedure for the determination of non-heme iron in tissue. Anal Biochem. 1967;18:559–63.
Imeryuz N, Tahan V, Sonsuz A, Eren F, Uraz S, Yuksel M, et al. Iron preloading aggravates nutritional steatohepatitis in rats by increasing apoptotic cell death. J Hepatol. 2007;47:851–9.
Garrick MD. Human iron transporters. Genes Nutr. 2011;6:45–54.
Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85.
Acknowledgments
This work was supported in part by a Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (19791054) and Grants-in-Aid from Nakayama Cancer Research Institute (Tokyo, Japan).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okada, K., Warabi, E., Sugimoto, H. et al. Nrf2 inhibits hepatic iron accumulation and counteracts oxidative stress-induced liver injury in nutritional steatohepatitis. J Gastroenterol 47, 924–935 (2012). https://doi.org/10.1007/s00535-012-0552-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-012-0552-9