Abstract
Background
Our previous studies demonstrated that p53-induced gene 11 (PIG11) was involved in arsenic trioxide (As2O3)-induced apoptosis in human gastric cancer MGC-803 cells. Here, we studied further PIG11 expression in human hepatocellular carcinoma (HCC) tissues and cell lines and compared the sensitivity to As2O3-induced cell apoptosis in HepG2 and L-02 cells.
Methods
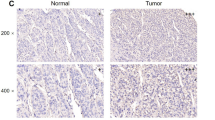
PIG11 expression in human normal liver tissues, HCC tissues, and cell lines was determined by immunohistochemistry and immunocytochemistry methods, using an anti-human PIG11 antibody. Cell viability was estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diplenyltetrazolium bromide (MTT) assay. Cell apoptosis was determined by flow cytometry. Reverse-transcriptase polymerase chain reaction (RT-PCR) and Western blotting were performed to analyze PIG11 mRNA and protein expression in cells. Protein intensity was calculated by comparison with the intensity of β-actin, using densitometry. PIG11 was knocked down using small interfering RNA (siRNA).
Results
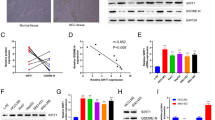
We found that PIG11 expression was significantly downregulated in HCC tissue and the cell lines (Bel-7402, SMMC-7721, HepG2 cells). Further, HepG2 cells were more sensitive to As2O3-induced apoptosis than L-02 cells. The expression of PIG11 mRNA and protein was upregulated to a greater extent in HepG2 than in L-02 cells. In the presence of actinomycin D or cycloheximide, the amount of PIG11 protein expression did not increase. Likewise, the inhibition of PIG11 by siRNA decreased As2O3-induced PIG11 protein expression by more than 85% and partially prevented As2O3-induced apoptosis in both HepG2 and L-02 cells.
Conclusion
The above results demonstrated that the PIG11 gene may be involved in As2O3-induced apoptosis in HepG2 cells and suggested that the adaptive response of PIG11 expression is one of the important factors in enhancing cell sensitivity to As2O3-induced apoptosis.






Similar content being viewed by others
References
Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein BA. Model for p53-induced apoptosis. Nature. 1997;389:300–5.
Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–51.
Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL. Galetin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J Biol Chem. 2002;277:3487–97.
Donald SP, Sun XY, Hu CAA, Yu J, Mei JM, Valle D, et al. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 2001;61:1810–6.
Gentile M, AhnstrÖm M, SchÖn F, Wingren S. Candidate tumor suppressor gene at 11q23-q24 in breast cancer: evidence of alteration in PIG8, a gene involved in p53-induced apoptosis. Oncogene. 2001;20:7753–60.
Zhu J, Jiang J, Zhou W, Zhu K, Chen X. Differential regulation of cellular target genes by p53 devoid of the PXXP motifs with impaired apoptotic activity. Oncogene. 1999;18:2149–55.
Woo SH, Park IC, Park MJ, Lee HC, Lee SJ, Chun YJ, et al. Arsenic trioxide induces apoptosis through a reactive oxygen species-dependent pathway and loss of mitochondrial membrane potential in Hele cells. Int J Oncol. 2002;21:57–63.
Ricketts SL, Carter JC, Coleman WB. Identification of three 11p11.2 candidate liver tumor suppressors through analysis of known human genes. Mol Carcinog. 2003;36:90–9.
Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999;35:1258–63.
Miller WH, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002;62:3893–903.
Murgo AJ. Clinical trials of arsenic trioxide in hematologic and solid tumors: overview of the National Cancer Institute Cooperative Research and Development Studies. The Oncologist. 2001;6(Suppl 2):22–8.
Liang XQ, Cao EH, Zhang Y, Qin JF. p-53-induced gene 11 (PIG11) involved in arsenic trioxide-induced apoptosis in human gastric cancer MGC-803 cells. Oncol Rep. 2003;10:1265–9.
Liang XQ, Cao EH, Zhang Y, Qin JF. A p53 target gene, PIG11, contributes to chemosensitivity of cells to arsenic trioxide. FEBS Lett. 2004;569:94–8.
Xiong XF, Li H, Cao EH. PIG11 protein binds to DNA in sequence-independent manner in vitro. Biochem Biophys Res Commun. 2007;358:29–34.
Chiba T, Yokosuka O, Fukai K, Kojima H, Tada M, Arai M, et al. Cell growth inhibition and gene expression induced by the histone deacetylase inhibitor, trichostatin A, on human hepatoma cells. Oncology. 2004;66:481–91.
Marshall KR, Gong M, Wodke L, Lamb JH, Jones DJ, Farmer PB, et al. The human apoptosis-inducing protein AMID is an oxidoreductase with a modified flavin cofactor and DNA binding activity. J Biol Chem. 2005;280:30735–40.
Park JW, Choi YJ, Jang MA, Baek SH, Lim JH, Passaniti T, et al. Arsenic trioxide induces G2/M growth arrest and apoptosis after caspase-3 activation and Bcl-2 phosphorylation in promonocytic U937 cells. Biochem Biophys Res Commun. 2001;286:726–34.
Liao WT, Chang KL, ChL Yu, Chen GS, Chang LW, Yu HS. Arsenic induces human keratinocyte apoptosis by the FAS/FAS ligand pathway, which correlates with alterations in nuclear factor-kappa B and activator protein-1 activity. J Invest Dermatol. 2004;122:125–9.
Barchowsky A, Roussel RR, Klei LR, James PE, Ganju N, Smith KR, et al. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol. 1999;159:65–75.
Ding WX, Shen HM, Ong CN. Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes. Hepatology. 2000;32:547–55.
Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal. 2005;7:1302–14.
Kim HJ, Chakravarti N, Oridate N, Choe C, Claret F-X, Lotan R. N-(4-Hydroxyphenyl) retinamide-induced apoptosis triggered by reactive oxygen species is mediated by activation of MAPKs in head and neck squamous carcinoma cells. Oncogene. 2006;5:2785–94.
Zhao S, Tsuchida T, Kawakami K, Shi CB, Kawamoto K. Effect of As2O3 on cell cycle progression and cyclins D1 and B1 expression in two glioblastoma cell lines differing in p53 status. Int J Oncol. 2002;21:49–55.
Rivera A, Maxwell SA. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 2005;80:29346–54.
Acknowledgments
This work was supported by grants from the National Science Foundation of China (30370386, 30570461) and the Hunan Provincial Natural Science Foundation of China (06JJ2048).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, XM., Xiong, XF., Song, Y. et al. Possible roles of a tumor suppressor gene PIG11 in hepatocarcinogenesis and As2O3-induced apoptosis in liver cancer cells. J Gastroenterol 44, 460–469 (2009). https://doi.org/10.1007/s00535-009-0030-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-009-0030-1