Abstract
Purpose
The impact of sarcopenia in oncology is increasingly recognized, yet little is known about its clinical implications in breast cancer. This systematic review and meta-analysis estimates the overall prevalence of sarcopenia in breast cancer, quantifies skeletal muscle index (SMI), and comprehensively evaluates sarcopenia’s impact on clinical outcomes.
Methods
We systematically searched primary original research published before June 2023 in four databases: the Cochrane Library via Wiley, CINAHL Plus with Full Text, Embase via Elsevier Excerpta Medica, and Medline via Ovid. Standardized mean SMI and 95% confidence interval (CI) were calculated by applying the random-effects model. The methodological quality of the included studies was assessed using the National Institutes of Health quality assessment checklist.
Results
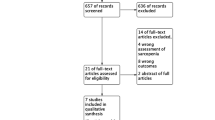
The systematic review included 17 studies with a total of 9863 patients; the meta-analysis included 12 of these studies. The mean prevalence of sarcopenia in breast cancer (stages I–III) was 32.5%. The mean SMI assessed by CT was 43.94 cm2/m2 (95% CI 42.87, 45.01; p < .01). Overall, low muscle mass was associated with chemotherapy toxicities, dose reductions, dose delays, or treatment discontinuation. Low muscle mass was generally associated with poor survival, but in some studies, this association was not significant or reversed direction.
Conclusion
Sarcopenia is not just a state of muscle mass loss, but an influencing factor on therapeutic effects and survival rates in oncology. It is thus necessary to recognize the risk of sarcopenia throughout the trajectory of cancer treatment, identify low muscle mass early, and manage it from a prehabilitation perspective.


Similar content being viewed by others
Data availability
Not applicable.
References
American Cancer Society (2023) Cancer Facts & Figures 2023. Atlanta: American Cancer Society https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf. Accessed 2 September 2023
Mattiuzzi C, Lippi G (2019) Current cancer epidemiology. J Epidemiol Glob Health 9(4):217–222
Iwase T, Wang X, Shrimanker TV, Kolonin MG, Ueno NT (2021) Body composition and breast cancer risk and treatment: mechanisms and impact. Breast Cancer Res Treat 186:273–283
Williams GR, Dunne RF, Giri S, Shachar SS, Caan BJ (2021) Sarcopenia in the older adult with cancer. J Clin Oncol 39(19):2068–2078
Au PC, Li HL, Lee GK, Li GH, Chan M, Cheung BM, Wong IC, Lee VH, Mok J, Yip BH, Cheng KK (2021) Sarcopenia and mortality in cancer: a meta-analysis. Osteoporos Sarcopenia 7:S28-33
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26(3):861–868
Aleixo GF, Williams GR, Nyrop KA, Muss HB, Shachar SS (2019) Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res Treat 177:569–579
Feliciano EM, Chen WY, Lee V, Albers KB, Prado CM, Alexeeff S, Xiao J, Shachar SS, Caan BJ (2020) Body composition, adherence to anthracycline and taxane-based chemotherapy, and survival after nonmetastatic breast cancer. JAMA Oncol 6(2):264–270
Aleixo GF, Valente SA, Wei W, Moore HC (2022) Association of sarcopenia with endocrine therapy toxicity in patients with early breast cancer. Breast Cancer Res Treat 196(2):323–328
Aleixo GF, Valente SA, Wei W, Chen PH, Moore HC (2023) Sarcopenia detected with bioelectrical impedance versus CT scan and chemotherapy tolerance in patients with early breast cancer. Breast Cancer 30(1):101–109
Pamoukdjian F, Bouillet T, Lévy V, Soussan M, Zelek L, Paillaud E (2018) Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: a systematic review. Clin Nutr 37(4):1101–1113
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S, Soerjomataram I (2022) Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast 66:15–23
Jang MK, Park S, Park C, Doorenbos A, Go J, Kim S (2023) Hematologic toxicities, sarcopenia, and body composition change in breast cancer patients undergoing neoadjuvant chemotherapy. Support Care Cancer 31(7):1–10
Ueno A, Yamaguchi K, Sudo M, Imai S (2020) Sarcopenia as a risk factor of severe laboratory adverse events in breast cancer patients receiving perioperative epirubicin plus cyclophosphamide therapy. Support Care Cancer 28:4249–4254
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
National Institute for Health and Care Research. International prospective register of systematic reviews (PROSPERO) https://www.crd.york.ac.uk/prospero/. Accessed 1 June 2023
National Heart, Lung, and Blood Institute, Study Quality Assessment Tools https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 2 July 2023
Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, Choi SK, Muss HB (2017) Body composition as a predictor of toxicity in patients receiving anthracycline and taxane–based chemotherapy for early-stage breast cancer. Clin Cancer Res 23(14):3537–3543
Caan BJ, Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw P, Quesenberry CP, Weltzien EK, Castillo AL, Olobatuyi TA, Chen WY (2018) Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol 4(6):798–804
Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J, Tubiana-Mathieu N (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26:861–868
Mazzuca F, Onesti CE, Roberto M, Di Girolamo M, Botticelli A, Begini P, Strigari L, Marchetti P, Muscaritoli M (2018) Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget 9(39):25714
Weinberg MS, Shachar SS, Muss HB, Deal AM, Popuri K, Yu H, Nyrop KA, Alston SM, Williams GR (2018) Beyond sarcopenia: characterization and integration of skeletal muscle quantity and radiodensity in a curable breast cancer population. Breast J 24(3):278–284
van den Berg MM, Kok DE, Posthuma L, Kamps L, Kelfkens CS, Buist N, Geenen M, Haringhuizen A, Heijns JB, van Lieshout RH, Los M (2019) Body composition is associated with risk of toxicity-induced modifications of treatment in women with stage I-IIIB breast cancer receiving chemotherapy. Breast Cancer Res Treat 173:475–481
Aleixo GF, Shachar SS, Deal AM, Nyrop KA, Muss HB, Chen YT, Yu H, Williams GR (2020) The association of body composition parameters and adverse events in women receiving chemotherapy for early breast cancer. Breast Cancer Res Treat 182:631–642
Hua X, Deng JP, Long ZQ, Zhang WW, Huang X, Wen W, Guo L, He ZY, Lin HX (2020) Prognostic significance of the skeletal muscle index and an inflammation biomarker in patients with breast cancer who underwent postoperative adjuvant radiotherapy. Curr Prob Cancer 44(2):100513
Aleixo GF, Valente SA, Wei W, Moore HC (2020) Association of sarcopenia with endocrine therapy toxicity in patients with early breast cancer. Breast Cancer Res Treat 196(2):323–328
Amitani M, Oba T, Kiyosawa N, Morikawa H, Chino T, Soma A, Shimizu T, Ohno K, Ono M, Ito T, Kanai T (2022) Skeletal muscle loss during neoadjuvant chemotherapy predicts poor prognosis in patients with breast cancer. BMC Cancer 22(1):1–11
Jang MK, Park S, Park C, Doorenbos AZ, Go J, Kim S (2022) Body composition change during neoadjuvant chemotherapy for breast cancer. Front Oncol 12:941496
Kwon MR, Ko ES, Park MS, Jeong WK, Hwang NY, Kim JH, Lee JE, Kim SW, Yu JH, Han BK, Ko EY (2022) Impact of skeletal muscle loss and visceral obesity measured using serial CT on the prognosis of operable breast cancers in Asian patients. Korean J Radiol 23(2):159–171
Liu X, Zhang E, Wang S, Shen Y, Xi K, Fang Q (2022) Association of body composition with clinical outcome in Chinese women diagnosed with breast cancer. Front Oncol 12:957527
Oliveira Júnior JC, Miola TM, Roman SM, Oliart-Guzmán H, Oliveira VS, Souza JD, Makdissi FB, Bitencourt AG (2022) Computed tomography assessment of body composition in patients with nonmetastatic breast cancer: what are the best prognostic markers? Radiol Bras 55:359–364
Tang R, Deng JP, Zhang L, Zhang WW, Sun JY, Chi F, Zhang J, Wu SG, He ZY (2022) Prognostic significance of the skeletal muscle index and systemic inflammatory index in patients with lymph node-positive breast cancer after radical mastectomy. BMC Cancer 22(1):1–9
Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE (2018) Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus 31(8):1–11
Trejo-Avila M, Bozada-Gutiérrez K, Valenzuela-Salazar C, Herrera-Esquivel J, Moreno-Portillo M (2021) Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 36:1077–1096
Yang Z, Zhou X, Ma B, Xing Y, Jiang X, Wang Z (2018) Predictive value of preoperative sarcopenia in patients with gastric cancer: a meta-analysis and systematic review. J Gastrointest Surg 22:1890–1902
Bozzetti F (2017) Forcing the vicious circle: sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann Oncol 28(9):2107–2018
Williams GR, Rier HN, McDonald A, Shachar SS (2019) Sarcopenia & aging in cancer. J Geriatr Oncol 10(3):374–377
Ubachs J, Ziemons J, Minis-Rutten IJ, Kruitwagen RF, Kleijnen J, Lambrechts S, Olde Damink SW, Rensen SS, Van Gorp T (2019) Sarcopenia and ovarian cancer survival: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle 10(6):1165–1174
Mintziras I, Miligkos M, Waechter S, Manoharan J, Maurer E, Bartsch DK (2018) Sarcopenia and sarcopenic obesity are significantly associated with poorer overall survival in patients with pancreatic cancer: systematic review and meta-analysis. Int J Surg 59:19–26
Kamarajah SK, Bundred J, Tan BH (2019) Body composition assessment and sarcopenia in patients with gastric cancer: a systematic review and meta-analysis. Gastric Cancer 22:10–22
Sun G, Li Y, Peng Y, Lu D, Zhang F, Cui X, Zhang Q, Li Z (2018) Can sarcopenia be a predictor of prognosis for patients with non-metastatic colorectal cancer? A systematic review and meta-analysis. Int J Colorectal Dis 33:1419–1427
Amini B, Boyle SP, Boutin RD, Lenchik L (2019) Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci 74:1671–1678
Sayer AA, Cruz-Jentoft A (2022) Sarcopenia definition, diagnosis and treatment: consensus is growing. Age Ageing 51(10):1–5
Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, Jang HC, Kang L, Kim M, Kim S, Kojima T (2020) Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21(3):300–307
Zanker J, Sim M, Anderson K, Balogun S, Brennan-Olsen SL, Dent E, Duque G, Girgis CM, Grossmann M, Hayes A, Henwood T (2023) Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J Cachexia Sarcopenia Muscle 14(1):142–156
Funding
This work was supported by a National Research Foundation of Korea grant (NRF No. 2021R1C1C2004628) funded by the government of South Korea (Ministry of Science and ICT).
Author information
Authors and Affiliations
Contributions
MKJ and SP performed title and abstract screening, full-text screening, data extraction, and quality assessment. MKJ and RR developed search strategies for this review, and RR made data-specific search strategies for each database. MKJ and CP applied meta-analysis and provided an interpretation of the findings. MKJ wrote the main manuscript text and prepared all the figures. AΖD and SK provided supervision for this project. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was a systematic review and meta-analysis of all published literature, and institutional review deemed this study exempt from full ethical review.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jang, M.K., Park, S., Raszewski, R. et al. Prevalence and clinical implications of sarcopenia in breast cancer: a systematic review and meta-analysis. Support Care Cancer 32, 328 (2024). https://doi.org/10.1007/s00520-024-08532-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00520-024-08532-0